Big news! China's first stem-cell therapy drug approved for market.
2025-01-03
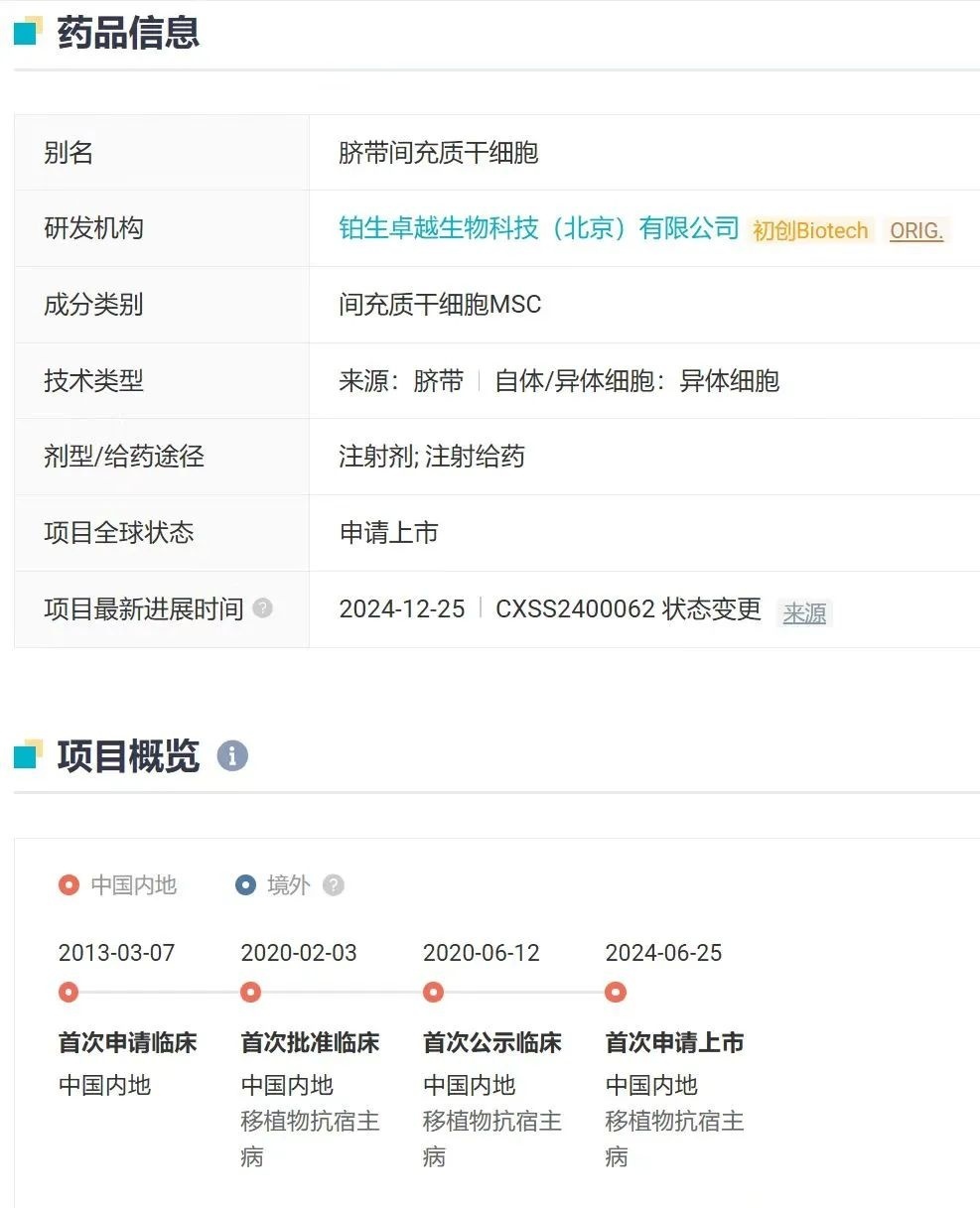
On January 2, the NMPA website announced that "AmyMaitose," a stem-cell therapy developed by Pusen Excellence Biotechnology (Beijing) Co., Ltd., has been approved for market launch. It is indicated for the treatment of acute graft-versus-host disease, primarily affecting the digestive tract, in patients aged 14 and older who have failed conventional hormone-based therapies. This is China's first Approved stem cell therapy for market release.

"Aimy Maitosai" (trade name: Ruibo Sheng) is a therapeutic product based on mesenchymal stem cells (MSCs), developed and clinically validated over many years. In March 2013, the company submitted an Investigational New Drug (IND) application for the product. However, due to the technical complexity of stem cell therapies and stringent regulatory requirements, the development process remained relatively slow. It wasn’t until June 2020 that "Aimy Maitosai" finally entered the clinical trial phase.
On June 12, 2024, Aimaitosai was placed under priority review and approval by the CDE, and on June 25 of the same month, it was officially submitted for NDA application, entering the acceptance queue. Notably, in August 2024, Beijing’s Drug Administration issued the nation’s first-ever stem cell "Drug Production License," a landmark achievement for Platinum Excellence. Then, on January 2, 2025, the drug received market approval, marking another significant milestone as a breakthrough therapy in its class domestically.
Currently, "AmyMaitosai" has completed its Phase II clinical trial, and the Phase III trial is now underway. In the exploratory Phase II trial, while the mesenchymal stem cell (MSC) group did not demonstrate a higher overall response rate (ORR) compared to the placebo group by Day 28, MSCs gradually showed therapeutic benefits after about two weeks on average. Notably, patients with intestinal involvement who completed all eight infusions appeared to derive significant benefits from the MSC treatment.
GVHD is a multi-organ syndrome that occurs after allogeneic hematopoietic stem cell transplantation, characterized by donor-derived lymphocytes attacking the recipient's tissues. It can affect various organs, including the skin, gastrointestinal tract, liver, lungs, and mucosal surfaces. GVHD is classified into acute GVHD (aGVHD), chronic GVHD (cGVHD), and an overlap syndrome exhibiting features of both types—serving as one of the leading causes of non-relapse mortality following allogeneic hematopoietic stem cell transplantation. Effective prevention and treatment of GVHD are crucial for the success of allogeneic HSCT, long-term post-transplant survival, and improving patients' quality of life.
On December 18, 2024, the U.S. Food and Drug Administration (FDA) approved Ryoncil (remestemcel-L-rknd), the first mesenchymal stromal cell therapy, for the treatment of steroid-refractory acute graft-versus-host disease (SR-aGVHD) in pediatric patients aged 2 months and older.
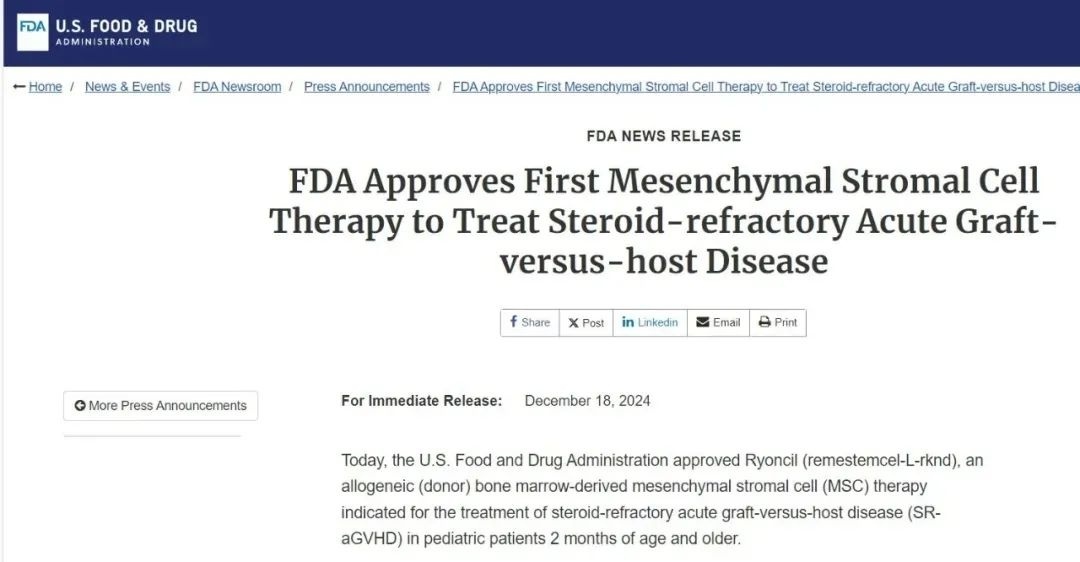
Emestemcel-L, developed by Mesoblast, was already approved for marketing in Canada, New Zealand, and Japan as early as 2012 and 2016. However, the journey to bring this drug to the U.S. market has been anything but smooth. The approval process there took a grueling 5.5 years, during which the company received two Complete Response Letters (CRLs). Despite numerous setbacks, the dedicated research team remained steadfast and ultimately succeeded in securing approval.
The approval of "Amy Maitosai" marks a significant milestone in China's stem-cell therapy field. This breakthrough not only brings hope to patients who urgently need effective treatment options, but also paves the way for stem cell therapy in our country. A significant step has been taken in the fields of clinical application and commercialization.
Related News




