China's first! Jiuzhitang Maker's stem-cell drug for autism officially begins clinical trials.
2025-08-27
On August 26, 2025, the kickoff meeting for the clinical trial evaluating human bone marrow-derived mesenchymal stem cell injection therapy for Autism Spectrum Disorder (ASD), conducted jointly by Jiuzhitang Maker (Beijing) Cell Technology Co., Ltd. (hereafter referred to as "Jiuzhitang Maker") and Beijing Anding Hospital, Affiliated to Capital Medical University (hereafter referred to as "Beijing Anding Hospital"), was successfully held in Beijing.
This is Jiuzhitang Maker's third domestically developed Class 1 biologic new drug based on stem cells—and also the first-ever clinical trial in China using human bone marrow-derived mesenchymal stem cells to treat Autism Spectrum Disorder (ASD).
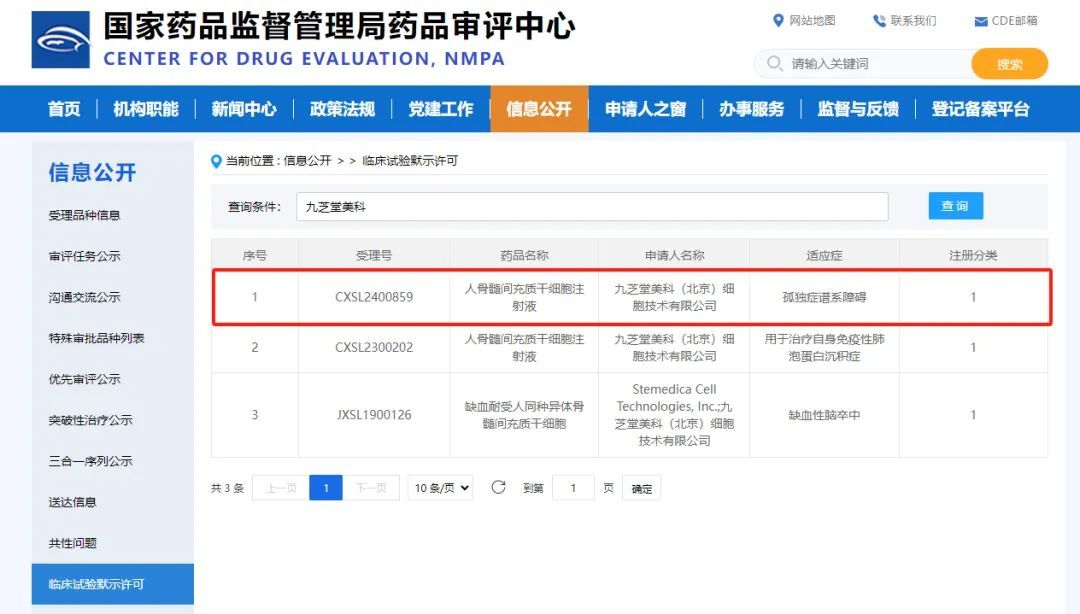
This clinical trial is an open-label Phase I study evaluating the safety and preliminary efficacy of human bone marrow mesenchymal stem cell (hBMMSC) injection in treating autism. The investigational drug, a human bone marrow mesenchymal stem cell (hBMMSC) injection, was independently developed by Jiuzhitang Maker. It is derived from the bone marrow of healthy, young adult donors and has been cultured throughout the process under low-oxygen conditions that closely mimic the real physiological environment of the human body. As a Class I biologic product, this innovative therapy aims to offer a groundbreaking treatment option for patients with Autism Spectrum Disorder (ASD).
Autism Spectrum Disorder (ASD), also known as autism, is a complex neurodevelopmental condition that typically emerges in early childhood. It is characterized by challenges in social communication, as well as repetitive behaviors and narrowly focused interests. According to the "Notice on Issuing the Trial Standards for Screening and Intervention Services for Autism Among Children Aged 0–6," released in August 2022 by the General Office of the National Health Commission, the prevalence of autism among children in China is approximately 7 per 1,000—meaning that about 7 out of every 1,000 children may be facing autism-related challenges. Meanwhile, U.S. data from 2020 revealed an overall autism prevalence rate of 2.76% among 8-year-old children, with boys being 3.8 times more likely to be diagnosed than girls. Currently, there are no approved medications in China specifically targeted to treat ASD, making the development of safe and effective therapies an urgent and unmet clinical need.
This project is led by Professor Wang Gang, Director of Beijing Anding Hospital Affiliated to Capital Medical University, Director of the National Mental Health Center, and Director of the National Clinical Research Center for Mental and Psychological Disorders. The team boasts profound academic expertise and extensive clinical experience in various fields, including depression, bipolar disorder, schizophrenia, anxiety disorders, and psychopharmacology.
Professor Wang Gang, the principal investigator of the project on-site, led the medical expert team in providing a detailed introduction to the clinical trial protocol for Jiuzhitang Maker’s new stem-cell therapy targeting Autism Spectrum Disorder (ASD). The presentation covered key aspects such as the study background, inclusion and exclusion criteria, group allocation design, and measures to protect participants' rights. Attendees from all parties engaged in an in-depth and thorough discussion about the scientific rigor, ethical considerations, and regulatory compliance of the study plan, ensuring the safety and efficacy of the trial.
Professor Wang Gang stated that he will now lead the expert team to meticulously and professionally engage in the clinical trial research on stem cell therapy for Autism Spectrum Disorder (ASD), in collaboration with Jiuzhitang Maker. He expressed hope that both parties will work together seamlessly, combining their strengths to firmly advance the project and jointly accelerate the translation of stem cell research findings into practical applications.
Zhang Quancheng, Chairman of Jiuzhitang Maker, stated: "Behind every child with autism lies a family enduring immense pressure and carrying high hopes. They face not only the challenges posed by the condition itself but also significant hurdles in social integration, education, and shaping their future lives. This profound societal impact underscores our deep sense of responsibility. At Jiuzhitang Maker, we are committed to transforming cutting-edge stem-cell technology into innovative therapies that truly benefit patients. Launching this clinical trial for a new autism treatment isn’t just a scientific breakthrough—it’s a heartfelt promise: We aim to harness the power of modern science to light up a beacon of hope for these extraordinary children and their families."
This strategic collaboration between Jiuzhitang Maker and Beijing Anding Hospital will seamlessly integrate Beijing Anding Hospital’s profound clinical expertise with Jiuzhitang Maker’s advanced stem cell production technology and robust quality management system, jointly paving the way for a groundbreaking approach to treating Autism Spectrum Disorder (ASD) using stem cell therapy. Leveraging their complementary strengths, both parties will adopt a rigorous scientific mindset and embrace an innovative spirit to accelerate clinical research, systematically evaluating the potential of stem cell therapy in enhancing patients’ social, cognitive, and behavioral functions. We are confident that this pioneering partnership will set a new industry benchmark in autism treatment, offering tangible hope and light to families worldwide while fostering a deeper integration of medical advancement and compassionate care.
Previous:
Related News




