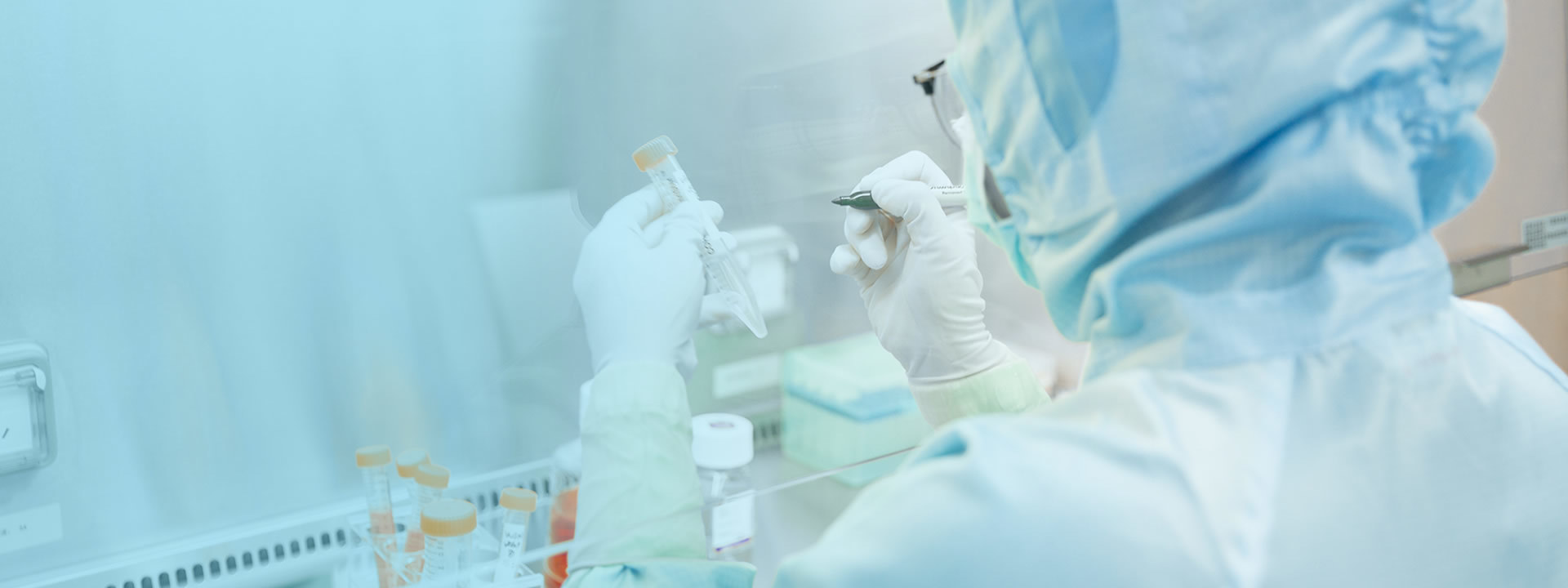
Production System
Jiuzhitang Maker has established a large-scale stem cell R&D and manufacturing facility in China Pharma Valley, compliant with China’s NMPA, the EU’s EMA, and the U.S. FDA GMP standards. The facility boasts an office and production area of approximately 4,800㎡, featuring four independent B+A clean zones on its production platform—enabling the manufacture of clinical-grade stem cells that meet both Chinese and U.S. regulatory requirements for drug submissions.
Special low-oxygen production process
Jiuzhitang Maker's unique hypoxic production process mimics the body's natural microenvironment, resulting in stem cells that exhibit enhanced homing ability, proliferative capacity, tissue-repair potential, ischemia tolerance, and improved inflammation-regulating properties.
Efficient large-scale production technology
Jiuzhitang Maker has introduced stem cell technology from the U.S.-based Stemedica Cell Technologies, further upgrading and refining its production processes and quality systems. This move addresses critical bottlenecks in the standardized production and large-scale manufacturing of stem cells, ensuring stable cell quality while enabling scalable, mass production.
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
COOKIES
Our website uses cookies and similar technologies to personalize the advertising shown to you and to help you get the best experience on our website. For more information, see our Privacy & Cookie Policy
These cookies are necessary for basic functions such as payment. Standard cookies cannot be turned off and do not store any of your information.
These cookies collect information, such as how many people are using our site or which pages are popular, to help us improve the customer experience. Turning these cookies off will mean we can't collect information to improve your experience.
These cookies enable the website to provide enhanced functionality and personalization. They may be set by us or by third-party providers whose services we have added to our pages. If you do not allow these cookies, some or all of these services may not function properly.
These cookies help us understand what you are interested in so that we can show you relevant advertising on other websites. Turning these cookies off will mean we are unable to show you any personalized advertising.
Copyright © Jiuzhitang Maker (Beijing) Cell Technology Co., Ltd.
Powered by: 300.cn SEO | Privacy Policy