Jiuzhitang Maker: China's First Clinical Trial Approved for a Stem-Cell Drug Targeting Autism!
2025-03-12
It is precisely in response to the urgent call of this societal issue that Jiuzhitang Maker, a national high-tech enterprise specializing in stem-cell drug research and development, has resolutely embraced the mission entrusted by the times. Drawing on the deeply rooted expertise and strong sense of social responsibility of Jiuzhi堂, a century-old pharmaceutical company (stock code: SZ000989), Jiuzhitang Maker has identified autism as one of its key research priorities. Stock code: SZ 000989) subsidiary Jiuzhitang Maker (Beijing) Cell Technology Co., Ltd. (hereinafter referred to as "Jiuzhitang Maker") has achieved a major breakthrough, successfully obtaining approval from the National Medical Products Administration (NMPA). As a result, Jiuzhitang Maker has become the first company nationwide authorized to initiate clinical trials for a new stem-cell therapy targeting Autism Spectrum Disorder (ASD). This milestone marks another groundbreaking step for Jiuzhitang Maker in the field of regenerative medicine, while also highlighting the company's robust R&D capabilities and promising growth prospects.
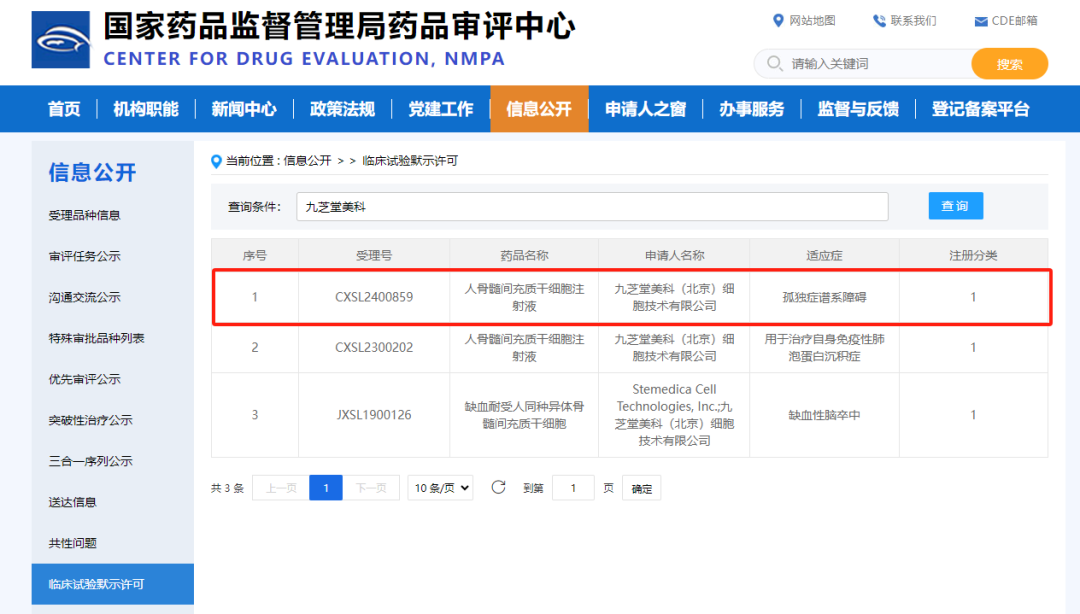
Basic Information on the Medication
Drug Name: Human Bone Marrow Mesenchymal Stem Cell Injection
Indications: Autism Spectrum Disorder (ASD)
Registration Category: Class 1 Therapeutic Biological Product
Application Item: Clinical Trial for Registration of Pharmaceuticals Manufactured Domestically
Applicant: Jiuzhitang Maker (Beijing) Cell Technology Co., Ltd.
Application Number: CXSL2400859
Jiuzhitang Maker’s human bone marrow mesenchymal stem cell injection is a cryopreserved stem cell preparation independently developed by the company. Derived from the bone marrow of healthy, young adult donors, it is cultivated throughout the process under low-oxygen conditions that closely mimic the body’s natural physiological environment. Clinically, it is intended for the treatment of autism spectrum disorder (ASD).
Autism Spectrum Disorder and Treatment Principles
Autism Spectrum Disorder (ASD), also known as autism, is a complex neurodevelopmental condition that typically emerges in early childhood. It is characterized by challenges in social communication, repetitive behaviors, and restricted interests. The symptoms of autism vary widely among individuals, significantly impacting their daily lives and social functioning. While the severity and specific manifestations of autism differ from person to person, there is currently no cure or definitive treatment available.
Mesenchymal stem cells exert positive effects on individuals with Autism Spectrum Disorder (ASD) through multiple mechanisms. They promote the repair of damaged tissues, synthesize and release anti-inflammatory cytokines and growth factors that support cell survival. Additionally, they help improve gut-brain axis function by modulating gut microbiota balance, suppress aberrant activation of microglia, enhance neuronal proliferation, and restore the structural integrity of neurons and mitochondria. These actions collectively contribute to reducing neuroinflammation and oxidative stress responses, potentially alleviating ASD symptoms and enhancing patients' quality of life. However, further clinical trials are still needed to fully validate their safety and efficacy.
Project Research Background
Autism Spectrum Disorder (ASD) is showing a significantly concerning rise in prevalence worldwide. According to the "Notice on Issuing the Trial Standards for Screening and Intervention Services for Autism Among Children Aged 0–6," released by the General Office of the National Health Commission in August 2022, the prevalence of autism among children in China is approximately 7 per 1,000—meaning that out of every 1,000 children, about 7 may be facing the challenges of autism. Behind these statistics lies the immense pressure and hardship endured by countless individuals and families affected by the condition. Data from 11 U.S. sites published in November 2020 revealed that the overall prevalence of autism among 8-year-old children was 2.76%, with boys being diagnosed at a rate 3.8 times higher than girls. These figures serve as a stark reminder: autism not only robs individuals of their ability to engage in normal social interactions and communication but also makes daily life profoundly difficult. For families, caring for someone with autism often comes at the cost of personal career aspirations, social opportunities, and even their own well-being. Over time, the financial strain and emotional toll can feel like an unrelenting, suffocating burden that’s hard to escape. Despite ongoing efforts by the medical community to advance autism research, current treatment approaches remain limited and fall short of meeting the urgent need for more effective interventions—both for patients and their families.
It is precisely in response to the urgent call of this societal issue that Jiuzhitang Maker, a national high-tech enterprise specializing in stem-cell drug research and development, has resolutely embraced the mission entrusted by the times. Leveraging the deep-rooted expertise and strong sense of social responsibility of Jiuzhi堂, a century-old pharmaceutical company (stock code: SZ000989), Jiuzhitang Maker has identified autism as one of its key research priorities.
After years of dedicated development, Jiuzhitang Maker has accumulated extensive hands-on experience in areas such as stem cell preparation techniques and quality management systems. Its stem cell products have earned multiple prestigious national-level certifications and are widely favored and highly recognized by top-tier, nationally renowned Grade-III hospitals across China. To date, Jiuzhitang Maker has successfully secured approval for three stem cell IND (Investigational New Drug) applications—specifically in fields like neurological and respiratory diseases—and is rapidly gaining prominence in the stem cell drug development arena, emerging as a shining star in the industry.
In the future, Jiuzhitang Maker will continue to push the boundaries of stem cell technology applications, driving the parallel development of multiple R&D pipelines. Guided by a dual-strategy approach focused on "process and quality," we will remain committed to developing safer, more effective stem cell products—with an even more rigorous scientific mindset and cutting-edge technological tools.
Jiuzhitang Maker’s goal is not only to bring a new ray of hope to individuals with autism, but also to make a significant mark on China’s ambitious vision for stem-cell drug research and development—and their practical applications. By doing so, the company aims to profoundly improve the quality of life for autistic patients worldwide, while simultaneously driving medical advancements and fostering societal progress.
This is a journey of exploration into the essence of life—a challenging yet glorious expedition as Jiuzhitang Maker moves forward into the future with unwavering determination.
Related News




