Jiuzhitang Maker: Research-grade Stem Cells—We Sincerely Invite Distributors to Join Us
2025-09-05
Jiuzhitang Maker deeply understands the needs of scientific research, drawing on the century-old pharmaceutical company Jiuzhi堂’s rich heritage in drug development and its GMP-grade stem cell technology platform. We meticulously craft stem cell products for research purposes according to pharmaceutical standards—ensuring safety, efficacy, and consistent quality—from raw materials all the way through to comprehensive support services, empowering scientists with reliable tools for pioneering discoveries. The reliability of an experiment often begins with the stability of the cells. 。
Many research teams have invested significant time and funding upfront, only to encounter the "cell trap" at the most critical experimental stage— Stem cells of unknown origin, with low activity, poor stability, and weak differentiation potential. ……
Once these issues arise, they can range from minor delays to severely impacting paper publication and project advancement—potentially even holding back the entire research initiative. Even more troubling, these problems often surface during the later stages of the study. Retracing costs are high, and time is irreversible. 。
Jiuzhitang Maker deeply understands the needs of scientific research, leveraging the century-old pharmaceutical company Jiuzhi堂’s rich heritage in drug development and its GMP-grade stem cell technology platform. We meticulously craft stem cell products for research purposes according to pharmaceutical standards—ensuring safety, efficacy, and consistent quality—from raw materials all the way through to comprehensive support services, empowering scientists with reliable tools for groundbreaking discoveries.

Advantages of Research-Grade Stem Cell Products
We don’t do “simple supply”—we deliver End-to-end support—from the cellular level to research-backed solutions , leveraging pharmaceutical-grade standards to overcome common procurement challenges in stem cell research.
1. Inter-batch zero drift, with highly reproducible experiments
Our research-grade human mesenchymal stem cells, Each batch originates from healthy donors who have undergone rigorous screening. , closed-loop control throughout the entire production process;
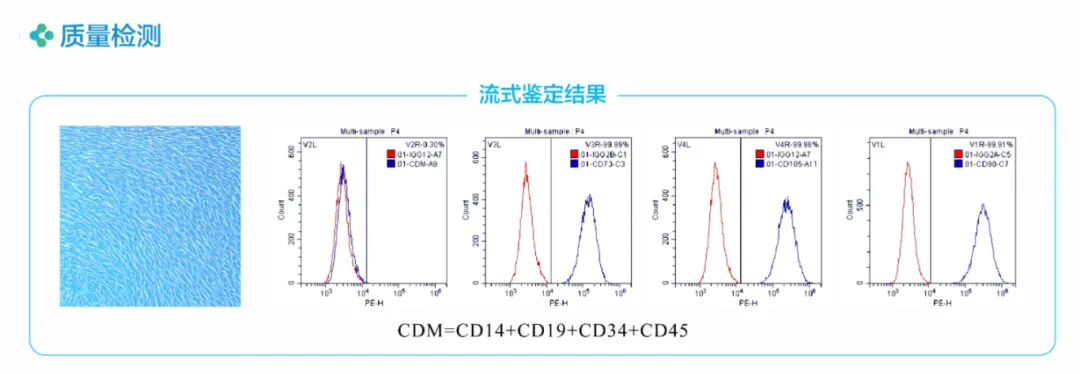
Key markers such as CD73, CD90, and CD105 are flow-cytometrically analyzed prior to shipment. Small batch-to-batch variation;
This means: Once the research team receives the cells, they can immediately finalize their experimental plan—no need to repeatedly troubleshoot issues like "sudden loss of cell function" or "systematic data drift." This approach completely eliminates the passive waste of reagents, consumables, and valuable time.
Cellular instability = a triple black hole of time, funding, and publications.
Address the issue of "cell stability" upfront,
It’s about getting the most cost-effective and comprehensive insurance coverage for the entire project.
2, Species are homologous, making experimental data more meaningful.
Numerous studies have shown that Mouse-derived cells differ significantly from human cells in aspects such as receptor-ligand profiles, metabolic enzyme systems, and the immune microenvironment. This often leads to insufficient persuasiveness when translating experimental results into clinical applications, typically requiring key validation using human-derived cells.
In recent years, The latest review trends in top journals like Nature and Cell also echo this pattern. : The editorial team and reviewers generally prioritize and encourage the supplementation of studies claimed to have "clinical relevance." Human cell data , to enhance the convertibility and credibility of the conclusions.
Using human-derived stem cells effectively resets the biggest variable— "species differences"—to zero, enabling more authentic experiments, stronger data, higher-quality publications, and faster translation into real-world applications.
3, Ready-to-use raw cell material—your trusted ally for exosome extraction
The research team working on the exosome project often feels utterly overwhelmed. "Juggling cell management in one hand while meticulously refining the extraction process with the other"—their manpower is essentially tied up at the cellular stage, leaving them only able to scratch the surface when it comes to truly optimizing and perfecting the core extraction techniques.
Ready-to-use raw material cells instantly shorten the process to "Goods Receipt - Collection" Two steps. Based on batch-stable cells, integrate the extraction process developed by the research group, The experiment cycle is shortened by at least 50%. , while also completely saying goodbye to the "all-or-nothing" risk posed by cell contamination.
The research team can channel all the released manpower into deeply exploring process parameters, ensuring that yield, purity, and functional validation are thoroughly addressed in one go—resulting in more robust data and making it easier to translate findings into impactful publications and patents.
Outsource "cell culture" to us, and you can focus entirely on "exosome science"—eliminating time, costs, and risks all at once, while accelerating your experimental progress to full speed.
4. Jiuzhitang Maker's Scientific Research Stem Cell Business Services
Jiuzhitang Maker is a stem-cell pharmaceutical company,
Only human-derived mesenchymal stem cells (MSCs) are provided.
Eliminate the risk of cross-contamination.
We provide a standard SOP for the culture of human-derived MSCs.
From recovery, inoculation, passage, harvesting to cryopreservation,
Can be directly filed into the lab SOP folder.
Company Advantages
When pursuing high-quality, reproducible, and translational scientific research, selecting a reliable stem cell supplier is crucial. Jiuzhitang Maker, with its established stem cell production technology and comprehensive stem cell quality management system, provides researchers worldwide with a solid foundation of assurance. Here are several key advantages of Jiuzhitang Maker’s research-grade stem cells:
1. Standard production platform ensures cell quality
International standard: , all test results were合格.
Cleanliness Assurance: Equipped with four independent Class B+A cleanrooms, the facility delivers a high-standard, closed-loop production environment with dynamic monitoring—perfect for manufacturing clinical-grade stem cell preparations that meet both U.S. and Chinese regulatory requirements.
Core process: Introduce and upgrade Stemedica's core U.S. production technology to break through bottlenecks in standardized stem cell manufacturing and large-scale production, ensuring consistent batch-to-batch quality and reliably delivering high-quality outputs at scale.
2, Hypoxic culture process enhances stem cell activity
Jiuzhitang Maker uses a low-oxygen environment for cultivation, mimicking the real physiological microenvironment inside the human body and significantly enhancing the biological activity of stem cells.
Stronger chemotactic ability: More sensitive response to inflammatory and repair factors such as EGF, bFGF, VEGF-121, and TNF-α;
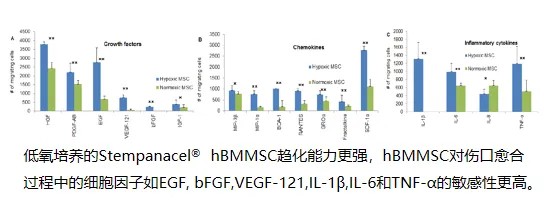
Superior functional features: Possessing enhanced homing, expansion, tissue repair, ischemia tolerance, and immunomodulatory capabilities;
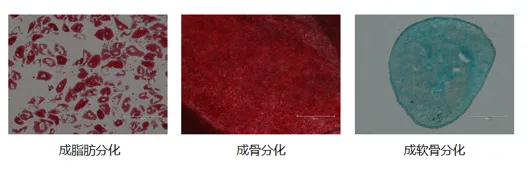
Higher differentiation potential: The osteogenic, adipogenic, and chondrogenic tri-lineage differentiation capability is stable and highly characteristic.
3, A full-cycle GMP quality system, backed by authoritative certification.
Jiuzhitang Maker has established a comprehensive GMP-quality management system that covers the entire lifecycle—spanning research and development, production, quality control, materials, documentation, and training.
Authoritative Certification
In October 2022, Jiuzhitang Maker launched four human bone marrow mesenchymal stem cell products derived from different donors. Fully tested by the NIFDC , all test results were合格.
In February 2023, Jiuzhitang Maker achieved certifications under ISO 9001, ISO 14001, and ISO 45001. International Trisolaris Standard Certification.
In March 2023, Jiuzhitang Maker was accredited by the China Association for Medical Biotechnology. "Certification for the Self-Regulatory Guidelines on Quality Management for Stem Cell Preparation." (The 5th nationwide)
In March 2024, the Quality Control Department of Jiuzhitang Maker Obtaining the accreditation certificate issued by the China National Accreditation Service for Conformity Assessment (CNAS) (Registration No. CNAS L20479), equipped with the necessary hardware, management standards, and testing capabilities that are both nationally and internationally recognized for mutual acceptance.
4. Clinical application validation boosts confidence in scientific research.
As of now, Jiuzhitang Maker's stem cell products have already undergone 3 clinical trials:
The first clinical trial for a stem-cell-based new drug (Acceptance No.: JXSL1900126) has now entered Phase IIa and is being conducted simultaneously at Beijing Tiantan Hospital, Peking University Third Hospital, and Beijing Tongren Hospital.
The second stem-cell new drug clinical trial (Acceptance No.: CXSL2300202) is being jointly conducted with the First Affiliated Hospital of Guangzhou Medical University. Currently, the project is in Phase IIa.
The third stem-cell new drug clinical trial (Application No.: CXSL2400859) is being conducted jointly with Beijing Anding Hospital, and the project has now entered Phase I.
Choose Jiuzhitang Maker's research-grade stem cells,
It's about choosing a research starting point that stands the test of time and is scientifically reliable.
We sincerely invite collaboration for a win-win future.
Jiuzhitang Maker is now opening up nationwide research and stem cell channel collaborations.
We sincerely invite distributors to jointly explore and build a new ecosystem for regenerative stem cell research.
We are looking forward to partners like these:
Specializing in the field of biological sciences, with access to resources from universities, research institutions, or CRO companies;
Possessing strong technical service capabilities and market promotion experience;
Recognizing the long-term value of high-quality research products.
Welcome to inquire
Phone: 18500271501 (Teacher Hu)
Email: lhhu@jztmaker.com
Official website: www.jztmaker.com
We look forward to partnering with Jiuzhitang Maker,
Entering the research arena with high-standard cell products,
Share in the benefits of regenerative medicine's growth!
Previous:
Related News




