People's Daily: The stem cell market is projected to surpass 25 billion yuan, with more than 30 companies already making strategic moves in this space.
2025-01-21
Stem cell therapy has been a hot area of research for many years, yet no products have yet been approved for market. On January 2, China's first stem cell drug received approval: the National Medical Products Administration granted conditional approval, via its priority review and approval process, to AmyMaitoCell Injection, developed by Pusen Excellence Biotechnology (Beijing) Co., Ltd., for the treatment of patients aged 14 and older whose hormone therapy has failed, particularly when gastrointestinal involvement is the primary issue. Acute Graft-versus-Host Disease 。
The stem cell market is demonstrating strong growth momentum. According to the "2024 China Stem Cell Industry Market Research Report" by Qianzhan Industry Research Institute, The Chinese stem cell market is projected to reach approximately 26.5 billion yuan in 2024, with the segment covering stem cell collection, preparation, and storage accounting for nearly 16 billion yuan of that total.
Although currently only one product has been approved domestically, there are still quite a few companies actively developing stem-cell therapies. According to an incomplete tally by the People's Daily Health Client, In 2024, more than 30 companies received approval for new drug clinical trials. , including Tianshi Li 、 Jiuzhitang Maker Several companies, including Qilu Cells and Quansheng Bio, are involved. Their therapeutic indications primarily cover diseases of the hematological system, cardiovascular system, nervous system, respiratory system, and autoimmune disorders.
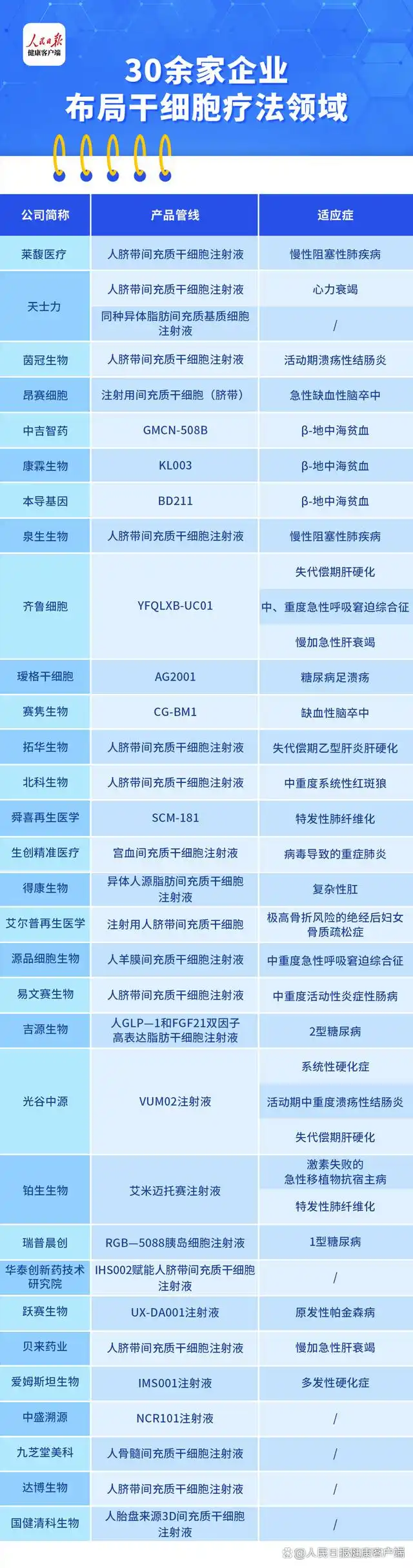
It is understood that the vast majority of these stem cell candidates are mesenchymal stem cells, including human ones. Umbilical Cord Mesenchymal Stem Cells , umbilical cord mesenchymal stem cells, adipose-derived mesenchymal stem cells, human amniotic membrane mesenchymal stem cells, bone marrow mesenchymal stem cells, and more. Among these, Human umbilical cord mesenchymal stem cells are the most prominent type, Such as Lefu Medical's human umbilical cord mesenchymal stem cell injection solution, Tianshi Li Human umbilical cord mesenchymal stem cell injection solutions, among others.
"Not all patients are suitable for stem cell therapy; the appropriateness of treatment must be evaluated based on each patient’s specific condition and medical status," said Hua Baolai, Director of the Department of Hematology at Beijing Century Tan Hospital, during an interview with reporters from the People’s Daily Health Client. He added that, as an emerging treatment approach, stem cell therapy requires close monitoring of its safety and efficacy in clinical applications to safeguard patients’ interests.
From the perspective of therapeutic efficacy, stem cells have shown promise across a variety of disease areas. For instance, in April 2024, Professor Hao Yin’s team from the Second Affiliated Hospital of Navy Medical University, in collaboration with Professor Xin Cheng’s team from the Center of Excellence in Molecular Cell Science at the Chinese Academy of Sciences, published their findings in the international academic journal *Cell Discovery*. Cell Discovery ) Announced the successful treatment of a Type 2 diabetes patient with severely impaired pancreatic function. This groundbreaking study marks the first-ever international application of autologous regenerative islet transplantation derived from stem cells, representing a major breakthrough in the field of diabetes therapy.
Related News




