Standing Out | Jiuzhitang Maker Named to Beijing’s “Specialized, Fine, and New” Enterprise List
2022-09-05
Recently, the Beijing Municipal Bureau of Economy and Information Technology released the "List of 'Specialized, Fine, and New' SMEs Identified in the Fourth Batch of 2022 in Beijing." Jiuzhitang Maker Successfully Selected 。
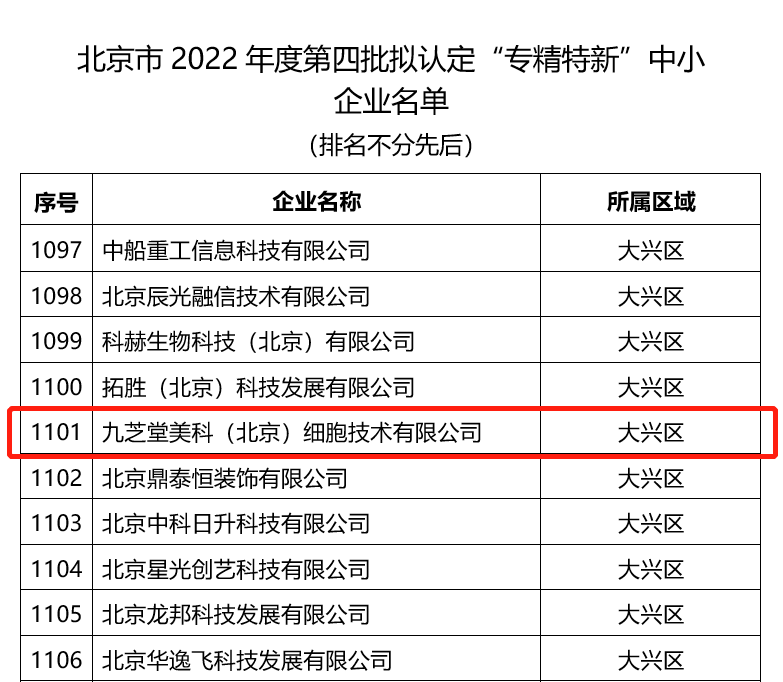
According to Beijing's eligibility criteria, companies selected as "Specialized, Fine, and New" enterprises must excel in specific areas such as economic performance, degree of specialization, innovation capability, and management practices. Achieve industry-leading standards , and is poised to become a globally leading industry in the future. Single-category champion or hidden champion enterprise 。
As a national high-tech enterprise, a Zhongguancun high-tech enterprise, and the drafting unit of the "Guidelines for Ethical Assessment of Stem Cell Sources," Jiuzhitang Maker has been named to Beijing's "Specialized, Fine, and New" enterprise list, a clear recognition of the company's comprehensive strengths—including its robust R&D investment in stem cells, advanced stem-cell expansion capabilities, significant market potential for stem-cell-based drugs, and outstanding innovation capacity.
Jiuzhitang Maker owns World-leading stem cell expansion technology , establishing a proprietary stem cell production and quality system, and has built a large-scale stem cell R&D and manufacturing facility in China Pharmaceutical Valley that meets cGMP standards set by China, the United States, and the European Union—producing Research-grade stem cell product line , Meets both U.S. FDA and China NMPA clinical trial requirements, It can be directly used in preclinical animal experiments, or further cultured and expanded under appropriate conditions for cell biology research or other scientific studies.

Additionally, Jiuzhitang Maker also offers Stem Cell CQDMO Services , offering stem cell-related services ranging from Pre-IND to commercial-scale drug production for hospitals, research institutions, and emerging companies.
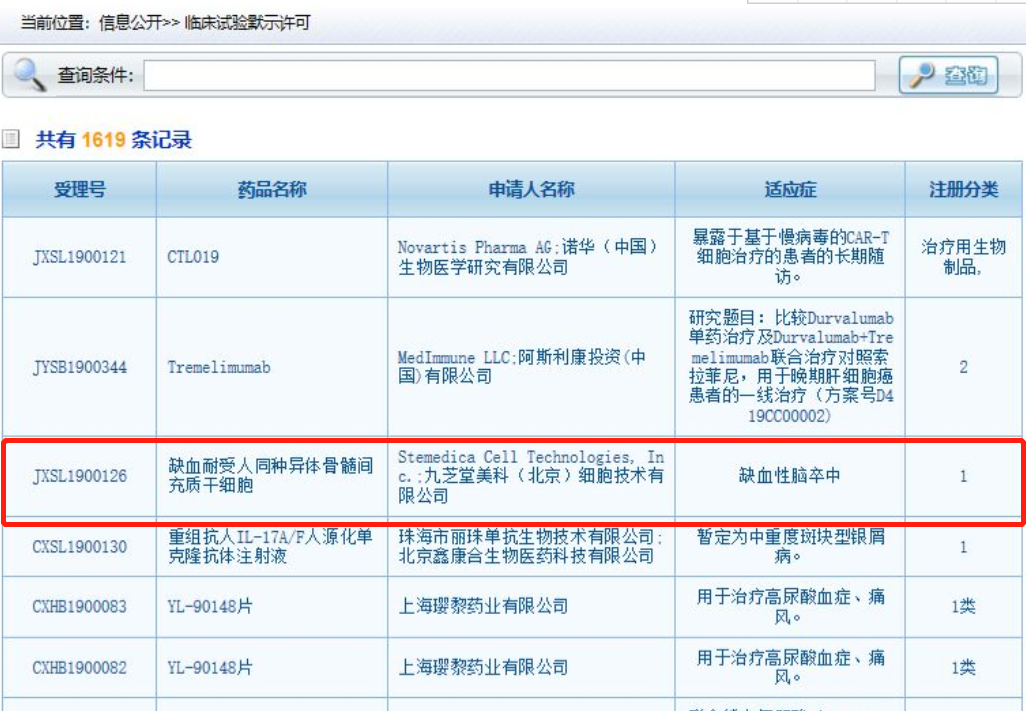
As of now, initiated by Jiuzhitang Maker China's first stem-cell therapy new drug for stroke The clinical trial has been approved and is currently being conducted in collaboration with Beijing Tiantan Hospital, Affiliated to Capital Medical University, to evaluate ischemic tolerance of allogeneic human bone marrow mesenchymal stem cells (it-hMSC) for the treatment of ischemic stroke. Phase I/IIa Clinical Trial 。
Not only that, Jiuzhitang Maker also makes a strong impact. Integrate global resources , collaborating with countries such as the United States and Kazakhstan, as well as domestic institutions like Beijing Tiantan Hospital, affiliated with the Capital Medical University Multiple Grade-III hospitals Establishing deepened collaboration. In response to the Belt and Road Initiative, Jiuzhitang Maker has joined forces with Kazakhstan’s ALTACO Company to jointly build an international medical center focused on scientific research and clinical translation studies centered around ischemia-tolerant mesenchymal stem cells. While committed to building a "Healthy China," this partnership blends Eastern and Western wisdom, using stem cells as a bridge to foster stronger ties between China and Kazakhstan.
In the future, Jiuzhitang Maker will fully leverage its internationally advanced R&D, production technologies, and industrialization capabilities to achieve more efficient global resource collaboration, accelerating the development and commercialization of stem-cell-based drugs while driving high-quality growth in the stem-cell industry—and ultimately playing a more proactive role in safeguarding public health.
Previous:
Related News




