Jiuzhitang Maker makes a strong appearance at the 6th International Conference on Immunogenetics and Cell Therapy
2022-09-01
From August 30 to 31, 2022, the IGC 2022 Sixth International Conference on Immuno-Gene and Cell Therapy, hosted by the Chinese Society of Biotechnology, was successfully held at the Beijing International Conference Center. As a vital force in the field of cellular biomedicine, Jiuzhitang Maker was proudly invited to attend this prestigious event, where it joined over 100 leading industry experts, top KOLs, and elite professionals for an exclusive gathering—a high-level forum that seamlessly blended cutting-edge science with robust industry insights.

This year's conference features four main venues and hosts 14 specialized forums, focusing on the latest policies and regulatory trends in immune cell therapy and stem cell treatments—both domestically and internationally. Discussions will also delve into cutting-edge topics such as off-the-shelf cell immunotherapies, stem cell-based gene therapies, and the therapeutic applications of iPSCs and MSCs, addressing current industry challenges while sharing forward-looking development insights and innovative approaches to technology translation.

Wisdom Collides · Forward-Thinking Insights
During the conference, representatives from various biotechnology companies—including Academician of the Chinese Academy of Sciences and Vice President of Capital Medical University, Wang Songling, as well as Dr. Gao Yansong, General Manager of Jiuzhitang Maker—delivered engaging presentations and shared valuable insights.
Among them, the Vice President of Jiuzhitang Group and Deputy General Manager of Jiuzhitang Co., Ltd., Dr. Gao Yansong, General Manager of Jiuzhitang Maker Titled "Several Reflections on the Pharmaceutical Development of Allogeneic Bone Marrow Mesenchymal Stem Cells," this presentation highlights the fundamental role and critical importance of stem cell expansion processes in the development of new drugs, as well as their impact on drug efficacy, clinical progress, and even business models. It also delves into the challenges faced in stem cell pharmaceutical development, analyzing them from four key perspectives: expansion capacity, process quality, cellular potency, and the identification of Critical Quality Attributes (CQAs). Furthermore, Jiuzhitang Maker shared its valuable experience in stem cell-based drug development—from environmental considerations to cell banking, raw material sourcing, and robust process control—earning high praise and recognition from the seasoned industry experts and academics in attendance.

Product Services · Highly Praised in the Industry
At this exhibition, as a National High-Tech Enterprise, Zhongguancun High-Tech Enterprise, and the drafting organization of the "Ethical Assessment Guidelines for Stem Cell Sources" Jiuzhitang Maker (Beijing) Cell Technology Co., Ltd. made a strong appearance, showcasing its unique features to the public. Research-grade stem cell product line , and Stem Cell CQDMO Services It has garnered significant attention and positive feedback from industry experts and leading figures in the field who were present at the event.


Jiuzhitang Maker has introduced stem cell technology from the U.S.-based Stemedica Cell Technologies, further refining and enhancing it to establish its own proprietary stem cell production and quality systems. The company has also built a large-scale stem cell R&D and manufacturing facility in China's Pharmaceutical Valley, which meets cGMP standards compliant with China, the United States, and the European Union. The facility produces Research-grade stem cell product line , and at the same time Meets US FDA and China NMPA clinical trial requirements , it can be directly used in preclinical animal experiments or further cultured and expanded under appropriate conditions for cell biology research or other scientific studies.

Additionally, Jiuzhitang Maker also offers Stem Cell CQDMO Services , offering stem cell-related services—from pre-IND submissions to commercial-scale drug production—for hospitals, research institutions, and emerging companies.
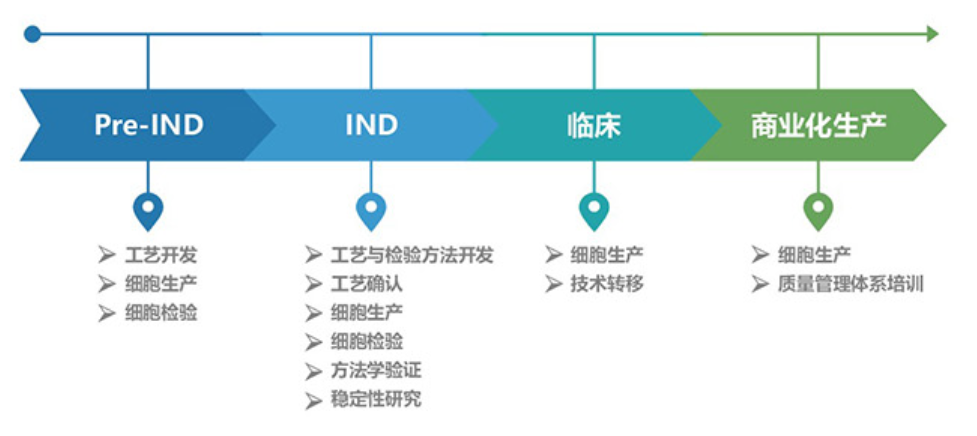
As of now, initiated by Jiuzhitang Maker China's first clinical trial for a new stem-cell therapy drug targeting stroke has been approved. Currently, we are collaborating with Beijing Tiantan Hospital, Affiliated to Capital Medical University, to conduct a Phase I/IIa clinical trial evaluating ischemic tolerance–human allogeneic bone marrow mesenchymal stem cells (it-hMSC) for the treatment of ischemic stroke.
Not only that, Jiuzhitang Maker has also powerfully integrated global resources, establishing deep collaborations with institutions such as universities and top-tier hospitals in the U.S., Kazakhstan, and China—including Beijing Tiantan Hospital, affiliated with the Capital Medical University. In response to the Belt and Road Initiative, Jiuzhitang Maker has jointly established an international medical center with Kazakhstan’s ALTACO Company, focusing on cutting-edge research and clinical translation centered around ischemia-tolerant mesenchymal stem cells. While actively contributing to the vision of building a "Healthy China," the company is bridging Sino-Kazakhstani ties by combining Eastern and Western medical wisdom, using stem cells as a vital link for mutual understanding and cooperation.
In the future, Jiuzhitang Maker will simultaneously advance both major disease areas and rare disease initiatives in its pipeline development. In therapeutic areas where mesenchymal stem cells and neural stem cells are applicable, such as neurological and respiratory disorders By fully leveraging world-class R&D, production technologies, and industrialization capabilities, we can achieve more efficient collaboration of global resources and expertise. Further expand future market opportunities and accelerate the rapid launch of pharmaceutical products. Furthermore, in the field of stem cell research aimed at decoding the secrets of life and health, Deepening the century-old brand value of Jiuzhitang , further accelerating the rapid development of regenerative medicine, particularly in the field of stem cells.
Previous:
Related News




