A Comprehensive Overview: Global Advances in the Stem Cell Industry and Clinical Translation
来源:Stem Cell Talk
2020-08-06
Cell therapy represents the third major pharmaceutical revolution, following small-molecule chemical drugs and large-molecule protein-based medicines—and is already making significant strides in treating and alleviating some of the most challenging and complex diseases, playing an increasingly vital role in the process. Stem cell therapy involves using stem cells or cells derived from stem cells, which are transplanted into the body via specialized techniques, to promote tissue and organ regeneration as well as facilitate overall recovery. This approach ultimately aims to treat or alleviate various medical conditions.
Stem cells, as a crucial component of cell therapy, play a vital role in organ repair and tissue regeneration, holding immense potential for future applications. This article will provide a brief overview of the global advancements in the stem cell industry and their clinical translation.
A Comprehensive Overview of the Global Stem Cell Industry
When it comes to the stem cell industry, a common description is that China's stem cell industry chain typically consists of three major segments: stem cell storage (upstream), stem cell expansion technologies and related drug development (midstream), and stem cell clinical applications (downstream).
Stem cell storage
Currently, the only globally recognized stem cell therapy is hematopoietic stem cell transplantation. Domestically, since 1999, China’s National Health Commission has introduced a series of policies and regulations governing cord blood hematopoietic stem cell banks, including the "Notice on Issuing the Planning for Establishing Cord Blood Hematopoietic Stem Cell Banks," released on May 20, 2001. However, this policy has repeatedly drawn criticism, primarily because the current "one province, one bank" management model fails to provide adequate regional coverage for most of China's population, falls short of meeting the growing demand for hematopoietic stem cell transplants, and suffers from inconsistent standards, making it difficult to ensure consistent quality. If we consider cord blood stem cell banks as part of the "national team," there are also several "grassroots teams"—private stem cell resource storage facilities—such as newborn stem cell banks that primarily store stem cells derived from umbilical cords and placental tissues, as well as adult immune cell banks focusing on peripheral blood immune cells. Additionally, there are specialized storage facilities for adipose-derived stem cells, dental pulp stem cells, urinary stem cells, and even uterine blood stem cells.
Stem Cell Drug Development
Although more than ten stem-cell drugs have already been approved globally (see Table 1), the U.S. FDA—known for having the highest standards in new drug regulation—has yet to approve a single stem-cell therapy to date (excluding orphan drugs and hematopoietic stem-cell products). The pioneering company in stem-cell drug development, U.S.-based Osiris, completed a failed Phase III clinical trial as early as 2009. Despite the FDA’s refusal to approve its product for market launch, technologies developed by Osiris have since been approved in Canada, New Zealand, and Japan. Currently, South Korea leads the world in the number of stem-cell drugs approved for commercial use. However, this may simply reflect the country’s regulatory policies rather than its actual R&D prowess in the field. After all, when it comes to pharmaceutical innovation, the U.S. FDA remains the gold standard for regulators worldwide. We must therefore remain humble, diligently studying and learning from the FDA’s rigorous approaches to continuously improve and strengthen our own capabilities.
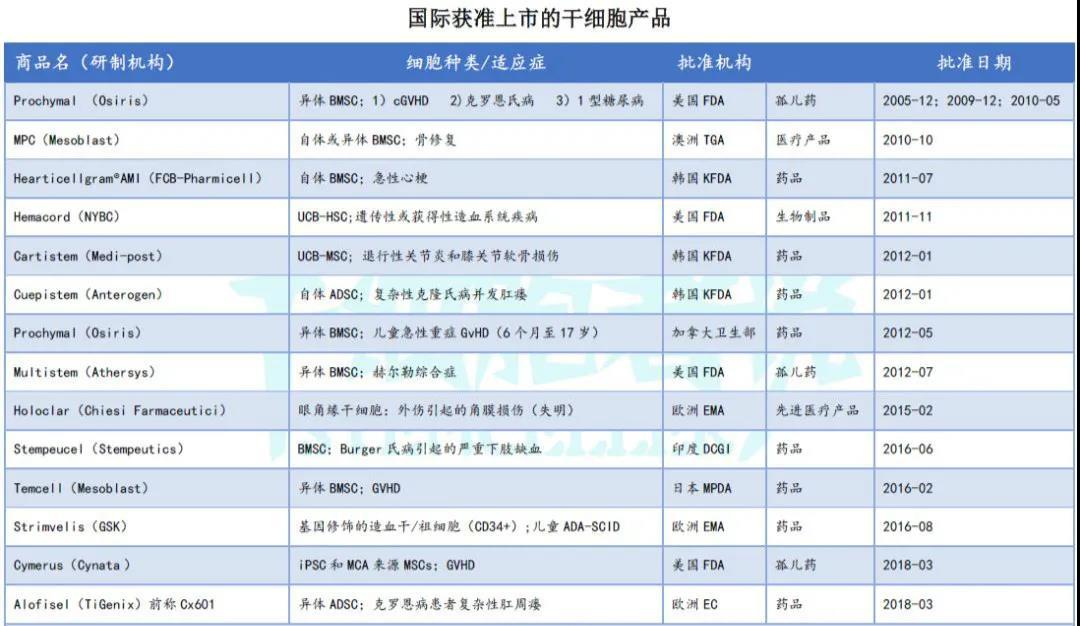
Table 1. Internationally Approved Stem Cell Products
In the field of stem cell research and development, China currently lacks a scientifically efficient approval system and robust regulatory policies with strict guidelines. At present, China’s stem cell therapies are gradually transitioning toward a "dual-track" approach—managed more like pharmaceuticals on one track and regulated as cutting-edge technologies on the other. Thus, when discussing clinical advancements in stem cell therapy in China, we can examine progress from two key perspectives: stem cell clinical research registration projects and ongoing clinical trials for new stem cell-based drugs. As of July 2020, 119 research institutions nationwide—including military hospitals—had successfully registered as clinical research facilities for stem cells through either the National Health Commission or the Military Commission’s Logistics Support Department. To date, a total of 86 stem cell clinical research projects have been officially approved for registration. These studies involve various types of cells, including embryonic stem cell-derived cells, neural stem cells, and mesenchymal stem cells sourced from diverse origins such as adipose tissue, umbilical cord, bone marrow, placenta, and menstrual blood. Notably, several of these clinical research initiatives have already made significant strides, paving the way for further innovation. Meanwhile, the door remains wide open for the submission of new stem cell-based drug applications.
In December 2017, the then-CFDA released the "Technical Guidance Principles for Research and Evaluation of Cell Therapy Products (Trial)," once again clarifying that cell-based products can be registered and regulated under the pharmaceutical review process. Currently, a growing number of such products are being submitted as drug applications. As of July 30, 2020, the Center for Drug Evaluation (CDE) has successively accepted 14 clinical trial applications for stem-cell-based drugs, with 11 of these having already received implicit approval. The approved indications include: knee osteoarthritis (3 studies), diabetic foot ulcers (1 study), rheumatoid arthritis (1 study), ischemic stroke (1 study), graft-versus-host disease (3 studies), inflammatory bowel disease (1 study), and idiopathic pulmonary fibrosis (1 study).
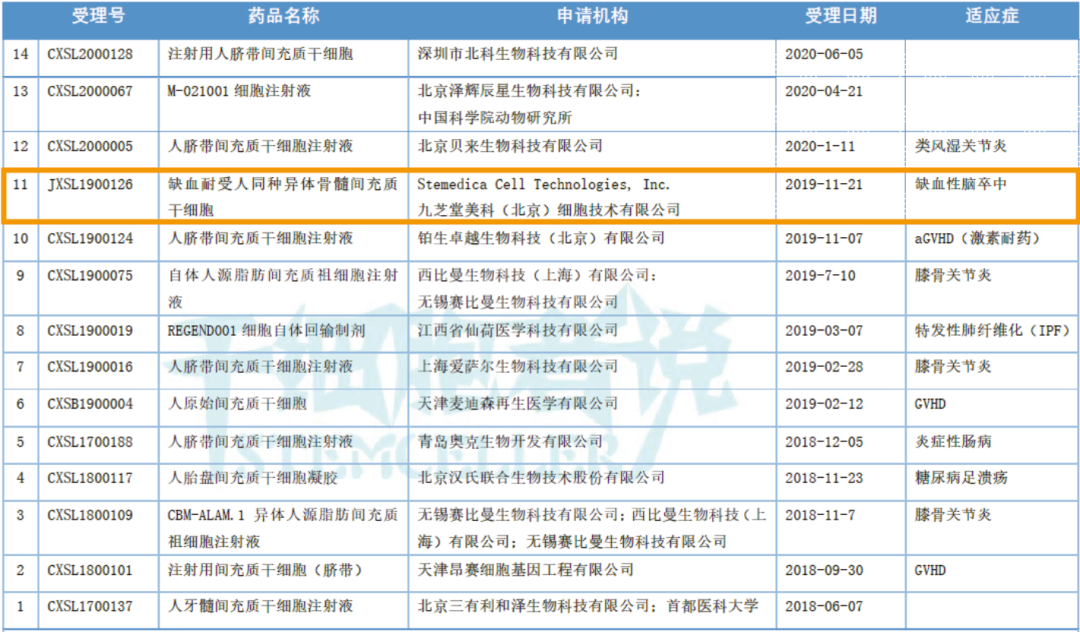
Table 2
We believe that in the future, more clinical research projects in China will complete their registration, and we’ll also see an increase in applications for stem-cell drug registrations—leading to the approval of clinical trial permits and ultimately paving the way for these products to be approved and launched onto the market.
A Global Overview of Clinical Translation Advances in Stem Cells
Hematopoietic stem cells
Hematopoietic stem cell transplantation for treating blood disorders is the earliest stem cell technology to be applied clinically—and currently remains the only stem cell therapy globally recognized and approved. Basic research on hematopoietic stem cells began in the 1940s, with the first successful transplant performed in the 1950s. By the 1960s, a robust system for HLA typing and matching had been established, and by the 1970s, hematopoietic stem cell technology had largely matured. Today, innovation continues in this field, including the "haploidentical transplantation" theory introduced by Chinese researchers, which has further lowered the barriers to successful matching and expanded the scope of applications for hematopoietic stem cells.
Mesenchymal stem cells
Besides hematopoietic stem cells, mesenchymal stem cells have currently made the most significant strides in clinical translation. As of June 20, 2019, there were 955 clinical studies related to mesenchymal stem cells registered on ClinicalTrials.gov, with 216 of them conducted in China (including Taiwan). These studies cover over a hundred different diseases, with neurological, cardiovascular, and orthopedic conditions emerging as the primary research areas. It’s important to note, however, that conducting clinical trials does not necessarily mean the technology is fully mature. Only after receiving regulatory approval and being officially classified as "stem cell drugs"—rather than mere stem cell products—can these therapies truly become safe and reliable treatment options for patients worldwide. Unfortunately, China has yet to approve any stem cell drug for market release.
Pluripotent Stem Cells
Pluripotent stem cells include embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC), both of which have the remarkable potential to differentiate into a wide variety of somatic cell types. The world’s first human embryonic stem cell line was established in 1998. In 2009, the U.S. FDA approved Geron Corporation to conduct the world’s first ESC-based clinical trial, using oligodendrocyte precursor cells derived from ESCs to treat spinal cord injuries; the first patient received treatment in 2010. Later that same year, the FDA also granted approval to Advanced Cell Technology (ACT) for a second ESC clinical trial, aiming to harness ESC-derived retinal pigment epithelial cells to combat age-related macular degeneration. In 2014, ViaCyte Inc. received FDA approval to initiate a clinical trial involving ESC-derived pancreatic beta cells for the treatment of diabetes. Although China started relatively late in this field, the ESC therapy area has garnered increasing attention in recent years. Academician Qi Zhou from the Institute of Zoology, Chinese Academy of Sciences, has spearheaded seven ESC-derived stem cell clinical research projects in humans (see Table 3). Notably, in 2017, Academician Zhou led China’s first-ever clinical study using ESCs to treat Parkinson’s disease. To date, ESC-based stem cell therapies being developed in China cover a range of conditions, including Parkinson’s disease, age-related macular degeneration, retinitis pigmentosa, primary ovarian insufficiency, and meniscus injuries, among others.
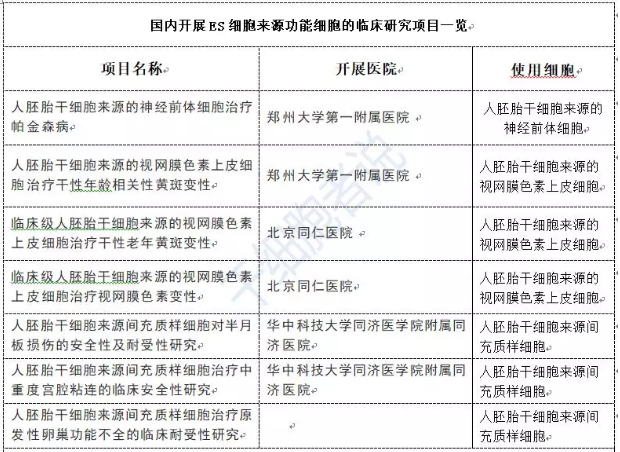
Table 3. Clinical Studies of ESC-Derived Functional Cells
Clinical research on iPSCs has seen governments around the world adopting a cautious, mostly wait-and-see approach. Japan, however, stands out as an exception, with various clinical studies leveraging iPSC technology currently in full swing (see Table 4). Since launching its first clinical trial using iPSCs to treat age-related macular degeneration in 2014, Japan has since approved allogeneic iPSC-based therapies for heart failure and Parkinson’s disease. The country’s first patient with Parkinson’s syndrome received treatment on November 9, 2018. Additionally, Japan recently granted approval to Keio University’s clinical research proposal titled "iPS Cell-Derived Neural Progenitor Cells for Spinal Cord Injury," with the first surgery already underway as of August 2019. Japan is steadily advancing in the clinical application of iPSCs, firmly positioning itself as a global leader in this cutting-edge field.
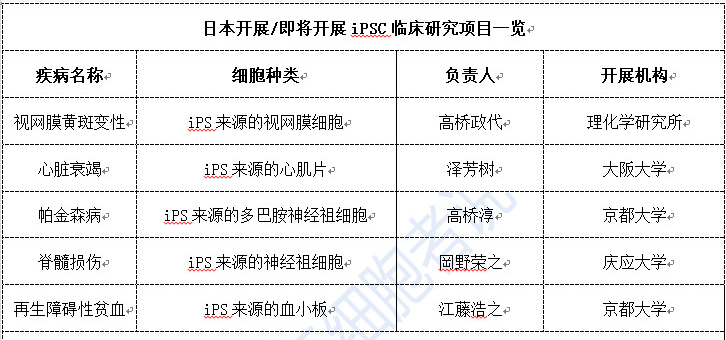
Table 4. Clinical Research Projects on iPSCs Conducted in Japan
The Future of the Stem Cell Industry
Currently, the role and value of stem cells in treating human diseases have begun to emerge clearly. In the field of regenerative medicine—particularly for major chronic illnesses and severe trauma repair—stem cell therapy has already proven to be an effective treatment option that addresses the limitations of conventional approaches.
In recent years, thanks to advancements in research and the gradual clarification of regulatory pathways, China's stem cell clinical translation has entered a new phase of development, prompting more patients to call for the clinical application of stem cells. From the perspective of the industry's long-term growth, engaging in both basic and clinical translational research is the only viable path toward building a comprehensive and sustainable stem cell industry chain—particularly as new drug development remains the most iconic business model in the pharmaceutical sector. As a result, stem cell-based drug research and development are gaining increasing recognition among industry professionals, attracting both capital and talent to this burgeoning field.
As scientists delve deeper into stem cell research, stem cells are poised to play an even greater role in treating diseases and enhancing health care in the future. The ongoing advancement of stem cell research will undoubtedly pave the way for a brighter, more promising future for humanity.
Related News




