Stem cells hold the promise of altering the "trembling fate" of Parkinson's patients, potentially freeing them from their condition.
2020-04-13
When Parkinson’s disease is mentioned, many people recall famous global figures such as former U.S. President Reagan, China’s mathematician Chen Jingrun, boxing legend Muhammad Ali, and Oscar-winning actress Katharine Hepburn. Despite their remarkable achievements and iconic legacies, they all faced the same devastating illness—Parkinson’s disease, a condition that not only gradually robs individuals of their ability to move but can ultimately lead to life-threatening complications.
About Parkinson's
Parkinson's disease (PD) is a common neurodegenerative disorder that primarily affects middle-aged and older adults, typically emerging after the age of 60. Symptoms include involuntary tremors in the hands, head, or mouth at rest, along with muscle rigidity, slowness of movement, and impaired postural balance—conditions that often make it difficult for patients to perform daily activities independently.
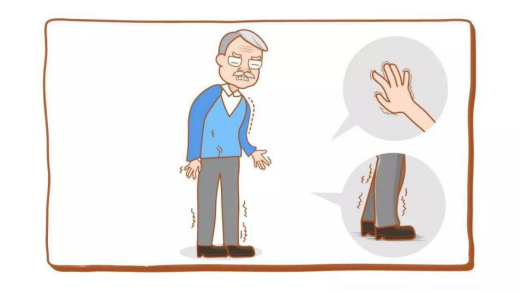
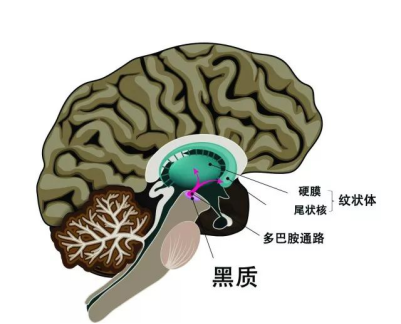
The primary pathological hallmark of Parkinson's disease is the progressive degeneration and loss of dopaminergic neurons in the substantia nigra of the midbrain, leading to reduced levels of dopamine (DA) in the striatum. Currently, the main treatment for Parkinson's disease remains pharmacological therapy [1]; however, medication alone fails to address non-motor symptoms such as mood disorders, sleep disturbances, dementia, and autonomic dysfunction, which can significantly impact patients' quality of life [2].
Over time, dopamine-producing neurons in the brains of Parkinson's disease patients gradually decline. Currently, treatments like levodopa can help compensate for the lost dopaminergic neurons, but only a small fraction of levodopa actually crosses the blood-brain barrier to exert its therapeutic effects—plus, it often causes significant side effects on the gastrointestinal and cardiovascular systems [3]. As a result, many scientists continue to search for more effective, functional therapies to tackle Parkinson's disease. Among these efforts, stem cells may hold the key to unlocking a breakthrough in Parkinson's treatment.
Stem cells bring hope for treating Parkinson's disease
With the rapid global advancement of stem cell research, stem cell technology has become a central focus in biological studies, increasingly demonstrating its immense potential across fields such as regenerative medicine, disease modeling, and drug screening.
Mesenchymal stem cells (MSCs) are widely available and can be isolated from sources including bone marrow, adipose tissue, peripheral blood, the placenta, amniotic fluid, umbilical cord blood, urine, and olfactory mucosa. Moreover, MSCs are multipotent stem cells with remarkable proliferative and differentiation capabilities. They are easily harvested, simple to isolate and culture, and offer advantages such as autologous transplantation without immunogenicity. These unique characteristics make MSCs a promising new approach for treating Parkinson’s disease.
The mechanisms by which MSCs treat Parkinson's disease are primarily linked to their replacement function, immunomodulatory effects, anti-apoptotic properties, and ability to enhance cellular metabolism.
Clinical studies confirm stem cell therapy for Parkinson's disease Safe and effective
In the late 1980s, scientists first initiated clinical trials using fetal brain tissue to replace lost dopamine-producing DA neurons, achieving promising clinical outcomes and demonstrating physiological dopamine release. As a result, stem cell therapy gradually gained recognition among researchers. With the successful establishment of various experimental models and the rapid advancement of clinical translation efforts, Parkinson’s disease treatment via stem cell therapy has attracted considerable attention. Today, stem cell therapy has progressed into clinical trial stages, yielding encouraging results thus far.
On November 13, 2017, International Stem Cell Corporation (ISCO), a U.S.-based biotech company developing stem cell therapies, announced positive interim 6-month results from its Phase I clinical trial evaluating the stem cell therapy ISC-hpNSC for Parkinson’s disease. All patients enrolled in the first cohort successfully met the primary study endpoint, and the treatment demonstrated a favorable safety profile.
In 2019, the journal *Cell Transplant* reported a clinical trial investigating the use of human neural progenitor cells (NPCs) as an intervention for Parkinson's patients. Eight Parkinson's patients underwent stereotactic surgery to directly inject NPCs into the dorsal striatum. Over a four-year follow-up period, seven of these patients were confirmed to have experienced a safe and effective treatment, with no signs of immune rejection or severe side effects. One year after stem cell intervention, all seven patients showed varying degrees of improvement in motor function, and five of them demonstrated enhanced responsiveness to existing therapies. Additionally, PET imaging revealed increased activity in midbrain dopaminergic neurons, indicating significant improvements in disease symptoms compared to pre-treatment levels.
On July 20, 2019, a clinical trial titled "Safety and Preliminary Efficacy Evaluation of Human Neural Stem Cell Therapy for Early-Onset Parkinson’s Disease With Motor Complications" was approved by the National Health Commission and registered with the Chinese Clinical Trial Registry. The study will employ an intranasal delivery method to conduct an interventional investigation in humans. Researchers administered intrathecal stem cell injections to 38 Parkinson’s patients and followed them for one month. Notably, all participants showed improvements in their UPDRS scores—lower scores indicating milder neurological deficits—as illustrated in the figure below. Additionally, patients experienced varying degrees of relief from motor symptoms, while assessments of mental, behavioral, and emotional functions also revealed significant reductions, suggesting that non-motor symptoms were similarly alleviated.
Stem cell drug development and applications: Meike is taking action.
In February 2020, Jiuzhitang Maker received approval for a clinical trial using imported bone marrow mesenchymal stem cells to treat ischemic stroke. The stem cell product employed in this trial is ischemia-tolerant allogeneic human bone marrow mesenchymal stem cells (ithMSC), manufactured by the U.S.-based company Stemedica. Stemedica produces its ithMSC products under fully controlled low-oxygen conditions, ensuring that their manufacturing processes and quality systems comply with U.S. cGMP standards and FDA requirements.
Jiuzhitang Maker (Beijing) Cell Technology Co., Ltd., as the sole Chinese partner for Stemedica Corporation’s stem cell technology, has acquired the core technologies enabling scalable, standardized, and traceable stem cell production by introducing Stemedica’s globally advanced clinical-grade stem cell preparation platform—filling a critical domestic gap. Moving forward, Jiuzhitang Maker will collaborate with Beijing Tiantan Hospital, Affiliated to Capital Medical University, to launch clinical trials aimed at treating ischemic stroke.
As the development and application of Meike stem cell therapies become increasingly mature, we will also focus on addressing more indications, ultimately benefiting a greater number of patients and contributing to China's healthcare sector.
References:
[1] ORIMO S. New advances in the diagnosis and treatment of Parkinson's disease [J]. Clinical Neurology, 2017, 57(6): 259-273.
[2] LINDVALL O. Clinical translation of stem cell transplantation in Parkinson's disease [J]. J Intern Med, 2016, 279(1): 30-40. [3] DJAMSHIDIAN A, POEWE W. Apomorphine and levodopa in Parkinson's disease: two revolutionary drugs from the 1950s [J]. Parkinsonism Relat Disord, 2016, 33 (Supplement 1): S9-S12.
Statement: The images in this article are sourced from the internet. If they infringe on any copyright, please contact us for removal.
Related News




