Jiuzhitang Maker Selected for Government's "COVID-19 Prevention and Control" Technology Transfer Project
2020-04-13
On February 5, 2020, to implement the requirements of the Municipal Party Committee and Municipal Government regarding scientific and technological efforts to combat the novel coronavirus (2019-nCoV)-infected pneumonia outbreak and to strengthen the district's systematic capacity to address emerging and sudden infectious diseases, the Daxing District Science and Technology Commission swiftly launched a call for science and technology projects focused on translating research findings into practical applications for COVID-19 prevention and control. Priority support will be given to areas such as rapid diagnostic kits for 2019-nCoV and related therapeutic drugs.
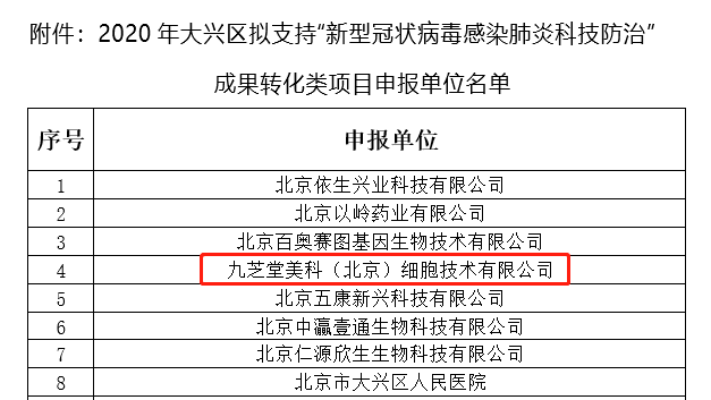
As a pioneer and leader in domestic stem-cell pharmaceuticals, Jiuzhitang Maker feels duty-bound to contribute to the fight against the pandemic. Actively responding to the government’s call, the company has mobilized its research teams to submit the project proposal titled "Mesenchymal Stem Cell Therapy from Bone Marrow for Treating Lung Damage Caused by Novel Coronavirus Pneumonia," which has now been successfully approved for funding.
Jiuzhitang Maker will conduct comprehensive preclinical research on bone marrow mesenchymal stem cell therapy for lung injuries caused by novel coronavirus pneumonia, adhering to the standards required for drug approval. The goal is to provide robust preclinical data—supporting both safety and efficacy—to underpin the clinical use of ischemia-tolerant allogeneic human bone marrow mesenchymal stem cells (it-hMSCs) in treating COVID-19 patients, while also generating relevant pharmacodynamic and pharmacological reports. In the future, once comprehensive preclinical data are obtained, these findings will be compiled into new drug application dossiers aimed at addressing lung damage induced by COVID-19.
According to reports, COVID-19 infection can trigger a robust inflammatory response in patients, leading to severe lung damage. Studies have shown that in most critically ill patients, the sudden onset of a cytokine storm may result in multi-organ failure, making it one of the key drivers behind the progression and fatal outcomes of COVID-19 pneumonia. [1-2] Mesenchymal stem cells (MSCs), known for their low immunogenicity, are capable of secreting growth factors, modulating immune and inflammatory responses, and combating oxidative stress—functions that enable them to directly aid in tissue repair and regeneration. [3] Recently, a clinical trial involving seven COVID-19 patients demonstrated that mesenchymal stem cell transplantation therapy could rapidly and significantly improve the prognosis of severely and critically ill patients, effectively mitigating the cytokine storm while showing no notable side effects. This breakthrough offers a promising new approach to the clinical management of COVID-19 patients. [4]
Jiuzhitang Maker’s ischemia-tolerant allogeneic human bone marrow mesenchymal stem cells (it-hMSC) are derived from the bone marrow of healthy, young adult donors and are cultured throughout the process under low-oxygen conditions that closely mimic the real physiological environment of the human body. Once introduced into the human body, these cells demonstrate significantly enhanced therapeutic efficacy. This stem cell product already boasts a strong clinical foundation—Jiuzhitang Maker has successfully submitted an application to the Center for Drug Evaluation (CDE) under China’s National Medical Products Administration to initiate clinical trials for ischemic stroke, and the application has been approved. This approval marks a critical milestone and sets the essential path for the future successful commercialization of this clinically-grade stem cell therapy.
Jiuzhitang Maker boasts a leading production platform and cutting-edge technologies in the stem cell field. Currently, it has established Beijing's first large-scale allogeneic stem cell R&D and manufacturing facility at the Daxing Biomedical Base in Beijing—known as "China's Pharmaceutical Valley"—which meets cGMP standards set by China, the United States, and the European Union.
References:
1. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al.: Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA, 2020.
2. Huang, C., et al., Clinical features of patients infected with the 2019 novel coronavirus in Wuhan, China. Lancet, 2020.
3. Zheng Sheng, Yang Juan, Tang Yingmei. The Role and Application Advances of Mesenchymal Stem Cells in Inflammation-Induced Immune Regulation [J]. Chinese Journal of Tissue Engineering Research, 2015, 019(045): P.7362–736
4. Leng Zikuan, Zhu Rongjia, Hou Wei, et al. Transplantation of ACE2- Mesenchymal Stem Cells Improves Outcomes in Patients with COVID-19 Pneumonia [J]. Aging and Disease, 2020, 11(2): 216-228.
Related News




