Jiuzhitang Maker Receives Funding Support from Daxing District to Boost the Development of the Pharmaceutical and Healthcare Industry
2020-04-09
Recently, Jiuzhitang Maker received financial support from Daxing District to boost the development of the pharmaceutical and healthcare industry. The project was selected following a review process conducted by the Daxing District Biomedical Industry Development Leading Group and the Daxing District Industrial Development Promotion Leading Group, in accordance with the "Provisional Measures for Promoting the Development of the Pharmaceutical and Healthcare Industry in Daxing District."
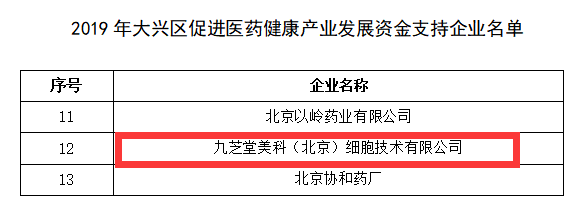
Jiuzhitang Maker's receipt of this round of funding reflects the Daxing District government's recognition of the company's technological capabilities and manufacturing strength, highlighting the company's potential to lead industry advancements in the stem-cell pharmaceutical sector.
Since 2018, Jiuzhitang Maker has been based in "China Pharmaceutical Valley"—the Beijing Daxing District Biomedical Base within the Zhongguancun Science Park—since which time Meike has rapidly advanced and achieved impressive results, earning recognition as a "Zhongguancun High-Tech Enterprise" among other accolades. "National High-Tech Enterprise" "Zhongguancun Golden Seed Enterprise" and titles such as "Technology-Based SMEs under the Ministry of Science and Technology," it has also received R&D expense support for technology-focused micro and small enterprises in the Zhongguancun Demonstration Zone, Beijing Municipal Major Science and Technology Project Funding 。
The company’s two projects—“Preclinical Study of Human Bone Marrow Mesenchymal Stem Cell Therapy for Acute Stroke” and “Mesenchymal Stem Cell Therapy for Lung Injury Caused by Novel Coronavirus Pneumonia”—have both been approved for funding.
Jiuzhitang Maker boasts a leading production platform and cutting-edge technologies in the stem cell field. In February 2020, Jiuzhitang Maker's stem-cell-based new drug has received approval from the Center for Drug Evaluation (CDE) of China's National Medical Products Administration for clinical trials. , this is currently the domestic The first one Clinical trials using imported stem cells are also currently underway in China. The only one Clinical trials using stem cells to treat major neurological conditions are of significant importance for the development of China's stem cell industry.
Moving forward, Jiuzhitang Maker will intensify its research efforts, accelerate the commercialization of scientific and technological innovations, and drive the R&D progress in the stem cell industry. This will provide critical technological support in the stem cell field, helping Daxing build the "China Pharmaceutical Valley" brand and establish a world-class biopharmaceutical base that is "nationally leading and internationally first-rate."
Related News




