Unveiling why stem cells may cause illness instead of healing!
2020-03-23
With the advancement of medical technology, stem cells are increasingly demonstrating their immense value in both theoretical exploration and clinical treatment, rapidly emerging as an effective tool to complement—or even replace—traditional therapies. From a broader perspective, stem cell technology is highly likely to spark a technological revolution and drive industry-wide upgrades in the biopharmaceutical sector.
But behind the immense potential and promise across so many directions, is stem-cell therapy safe? And what are the potential risks involved? How can these risks be effectively mitigated?
I dug out a highly informative and insightful lecture that perfectly answers this question. The lecture highlighted that stem cell preparation is an advanced, cutting-edge technology that demands utmost precision and rigor, involving sophisticated genetic engineering techniques like complex RNA transcription. Improperly prepared stem cells not only fail to deliver therapeutic benefits—they may even prove ineffective because the cells can’t reach the targeted diseased areas, potentially leading to new health issues instead.
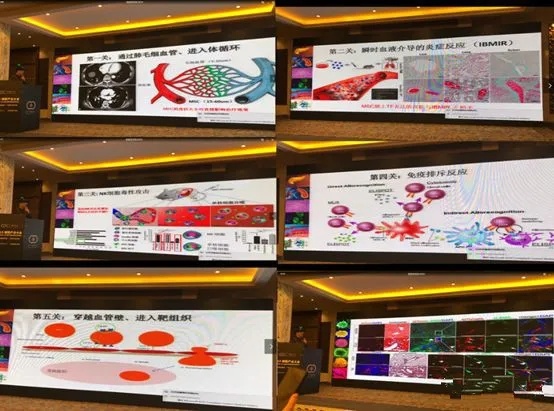
Possible diseases and their causes
1. Causes pulmonary embolism of varying degrees. (Some stem cells are too large to pass through the pulmonary capillaries.)
2. This leads to inflammation within blood vessels, triggering a blood clotting response. For example, blood clots may form in organs like the liver or lungs. (A certain tissue factor expressed by stem cells was not removed during in vitro culture.)
3. Not only does it fail to treat fibrosis, but it actually induces fibrosis instead. (When passaging stem cells using animal serum from different batches or of varying quality, consistency among the stem cells cannot be guaranteed, leading to their differentiation into other functional cell types, such as fibroblasts.)
4. May promote the growth of tumor tissue within the body. (Patients with existing tumors were enrolled without undergoing routine screening tests.)
5. Triggering an immune rejection response within the body (due to unstable culture conditions causing partial differentiation of stem cells).
The likelihood and reasons why stem cell therapy may be ineffective
1. The introduced stem cells are attacked by NK cells in the body, resulting in significant loss before reaching the target lesion site. (The stem cells' "immune passport" is lost during ex vivo culture.)
2. Stem cells delivered via intravascular infusion cannot effectively cross the vessel walls to reach the target tissue (due to low expression of receptors that mediate vascular penetration, and they fail to undergo selective activation during in vitro culture).
3. The stem cells provided lack sufficient activity, and once they reach the tissue, they fail to withstand the harsh microenvironment. For instance, in some patients, the microenvironment itself is already characterized by inflammation, microbial overgrowth, and an excess of reactive oxygen species—conditions that prevent the cells from surviving, let alone delivering therapeutic benefits. (Additionally, stem cell-related active components and anti-aging factors were not selectively activated during the culture process.)
Dr. Yin Qinwei, the expert lecturer featured in this video, is a leading authority on clinical stem cell preparation technologies. He is a recipient of the National Second-Class Award for Invention in the stem cell field, a fellow of the Chinese Academy of Sciences' "Hundred Talents Program," and a former member of the Systems Decision-Making Group at Harvard Medical School. Additionally, he currently serves as a distinguished professor at the Stem Cell Translation Center affiliated with Beijing Military Region General Hospital and Peking University Cancer Hospital.

In this presentation, Professor Yin first highlighted the safety and efficacy of mesenchymal stem cells, noting that they do not carry the potential risk of tumorigenicity seen with embryonic or induced pluripotent stem cells, while also demonstrating superior multi-directional differentiation capabilities compared to lineage-restricted stem cells like hematopoietic stem cells.
However, he emphasized that the key to ensuring the safety and efficacy of stem cell therapies lies in preparing high-quality, properly qualified stem cells. Using substandard or improperly processed cells, on the other hand, could actually lead to the development of new diseases—making this an issue that demands meticulous and serious attention. This is precisely why the outcomes of stem cell–related clinical studies worldwide remain inconsistent—and in some cases, even carry toxic side effects.
The report also outlines several challenges currently faced by mesenchymal stem cells, along with potential solutions.

Among other points, he specifically provided examples to highlight the major risks—ranging from significant health concerns to the emergence of entirely new diseases—that arise when globally produced stem cells fail to meet quality standards. Addressing these issues, he also proposed tailored solutions. In 2019, together with his research collaborators, he published a comprehensive article in a Nature sub-journal detailing standardized protocols for preparing mesenchymal stem cells, and for the first time introduced the concept of "empowered mesenchymal stem cells."

The field of stem cell therapy is a rising star, yet it’s inherently interdisciplinary. To ultimately achieve therapeutic success, experts from diverse fields—including microbiology, zoology, medicine, robotics, artificial intelligence, ergonomics, materials science, and even capital investment—must collaborate closely, leveraging their unique strengths in tandem.
At its core, the most critical factor is the preparation of high-quality, qualified stem cells—this is the foundational and most essential guarantee for both effective therapeutic outcomes and patient safety. This point has consistently been emphasized by regulatory bodies such as the National Medical Products Administration, the National Health Commission, and the China Institute of Drug and Biological Products Control, and it remains the key aspect scrutinized during clinical trial reviews.
Reprint Notice: This article is reprinted from MedShengHui, authored by Dr. Wang, a regenerative medicine expert.
Related News




