Make quality your belief—Meiko’s “Quality Month” Training
2020-03-16
Quality is the lifeblood of a company and the foundation of its reputation. By fostering a scientific understanding of quality management, enhancing employees' quality awareness, and supporting the continuous improvement of the company’s quality management system, we can ensure reliable quality assurance for our products.
For a pharmaceutical manufacturing company, quality is not only a matter of life or death—it’s also a critical issue that directly impacts human health!
The sudden outbreak of the pandemic delivered a severe blow to businesses' normal operations. How can companies turn this crisis into an opportunity? For corporate management, it’s a major test. During the 2003 SARS epidemic, Alibaba leveraged its exceptional leadership and strategic decisions to foster deep internal cohesion, enabling the company to make a transformative leap—from being merely excellent to achieving true excellence. This is a story that many organizations would do well to study and draw inspiration from.

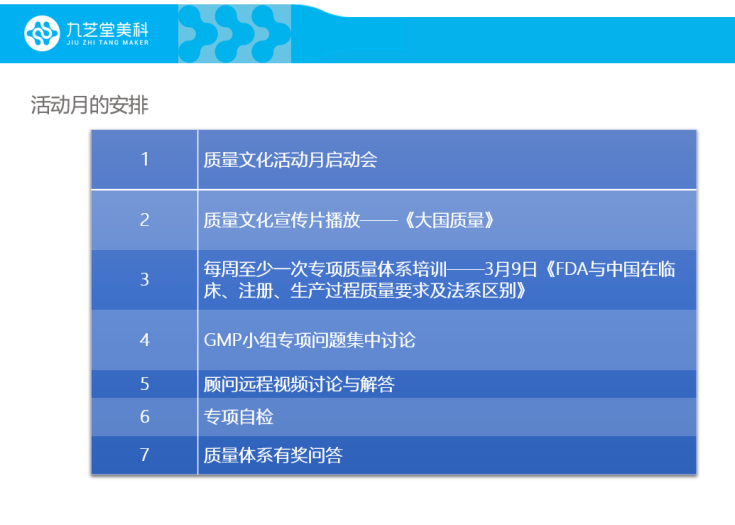
During this epidemic response period, as many operations were disrupted, Jiuzhitang Maker’s Quality Assurance Department, under the guidance of Chairman Zhang Quancheng and CEO Gao Yansong, launched a "Quality Activity Month" initiative. The goal was to reinforce the importance of quality management across all company departments, foster a deeper understanding of quality principles among every employee, and ultimately cultivate Meike’s unique quality culture.

During the first week of Quality Culture Month, Meike kicked off the event by holding a kickoff meeting via Zoom, sharing inspiring quality-related stories from everyday life—ranging from national and societal examples to vivid cases involving individual employees and businesses. The session encouraged staff to prioritize quality training and actively enhance their own learning in this area.
During the second week of Activity Month, Meike once again conducted a company-wide training session centered around the 2020 3.15 theme, "Uniting Our Strengths," with the focus on "Strengthening Quality Awareness and Enhancing Management Practices"—a direct response to the 3.15 campaign through concrete actions.

Meike plays the documentary "Quality of a Great Nation" for all employees.
Additionally, the company is also streaming the video "Quality of a Great Nation" online during the lunch break for all employees, enhancing both the atmosphere and the fun of Quality Culture Month.
Over the next two weeks, the company will continue to provide ongoing quality education to employees through various initiatives, including discussion sessions, promotional video screenings, remote advisory support for addressing specific issues, internal self-assessments, and engaging knowledge quizzes tied to the company’s quality culture—and all with prizes awarded for participation. Additionally, the team will conduct comprehensive briefings for everyone on key considerations related to drug development, clinical operations, manufacturing, and pharmacovigilance, aligned with the latest revisions of the Pharmaceutical Administration Law and significant new regulatory changes introduced by national drug oversight authorities.
"Consistent quality and progress can help a company achieve success, while even a single quality incident has the potential to ruin it. No one wants to produce defective products—not even those who may not fully understand what product quality truly means. Instead, everyone aspires to create excellent products with their own hands. Therefore, product quality isn’t just a matter of awareness; it’s fundamentally a question of responsibility—and at its core, that responsibility stems from our systems and processes. The purpose of Quality Month is to align everyone’s understanding of quality with the company’s overarching quality culture. After all, a quality system isn’t cold or impersonal; it doesn’t ignore human factors or show no mercy. On the contrary, quality embodies the simplest yet most essential principle: whether something is done right or wrong depends entirely on your personal accountability—not on what I say, what you say, or even what your supervisor says, but on what the system clearly demands of you." This is the philosophy that Meiko’s Quality Assurance Department has steadfastly upheld.
Jiuzhitang Maker is not only a pharmaceutical R&D company but also a sterile biopharmaceutical manufacturer—this responsibility behind the scenes compels the Meike team to prioritize quality above all else.
"The 19th National Congress Government Work Report clearly stated that China must complete its transformation from a manufacturing giant to a manufacturing powerhouse. Under the 'Made in China 2025' initiative—driven by innovation and prioritizing quality—Meiko will steadfastly uphold our core principle of 'Quality First, Integrity Rooted,' firmly embracing the guiding principle of fostering dual-engine momentum through R&D innovation and robust quality management. Through the collective efforts of every Meiko employee, we aim to make quality not just a standard, but a deeply held belief. After all, at the heart of quality lies fairness and equality for people—and it is people-centered. By continuously strengthening our company’s systems and processes, we will ultimately cultivate a quality culture where valuing quality means honoring personal responsibility and integrity, making it virtually impossible for those who neglect quality to succeed."
Previous:
Related News




