How close are stem-cell therapies for ischemic stroke to reaching the market?
来源:Meike
2019-08-22
Jiuzhi堂 is optimistic about the promising future of merging regenerative medicine with traditional Chinese herbal therapy. To seize this opportunity, Jiuzhi堂 launched the Zhuhai Hengqin Jiuzhi堂 Yonghe Qihang Fund, which has already invested in the U.S.-based company Stemedica. At the same time, Jiuzhi堂 established Jiuzhitang Maker—the sole Chinese partner entrusted with managing Stemedica’s cutting-edge stem-cell technology in China.
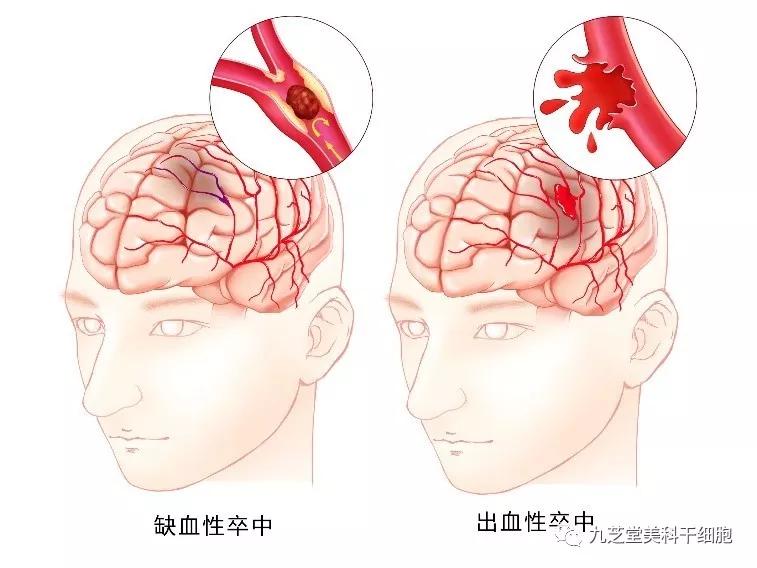
Stroke encompasses two main types: ischemic stroke (cerebral infarction) and hemorrhagic stroke (cerebral hemorrhage, or brain bleeding). It is a condition characterized by the death of brain cells and tissues. Among these, ischemic stroke accounts for more than 75% of all strokes and remains the primary type.
Currently, stroke treatment primarily focuses on the acute phase; however, once six months have passed, recovery outcomes significantly decline—or even come to a halt—leaving patients with permanent disabilities of varying degrees. Aside from physical therapy, there are virtually no pharmacological interventions available to aid patients in their recovery.
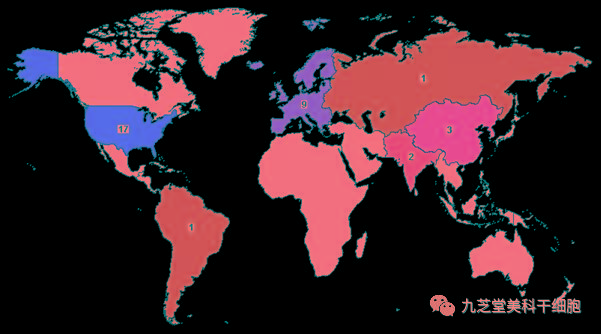
Stem cells have shown promising potential as a specialized treatment for stroke. According to data from the ClinicalTrials.gov website, as of now, a total of 35 Phase I, II, and III clinical trials evaluating stem cell therapy for ischemic stroke have been conducted worldwide—17 in the United States, 9 in Europe, 3 in China, and 6 in other countries.
Geographic Distribution Map of Clinical Trials for Stem Cell Therapy in Ischemic Stroke
Data shows that most clinical trials of stem cell therapy for stroke are still in Phase I or Phase II, with only one having advanced to Phase III and just beginning patient recruitment.

A new drug typically undergoes a lengthy and challenging journey—from initial concept to eventual market launch, which involves several critical stages: basic research, drug discovery, preclinical studies, and finally, clinical trials (including the aforementioned Phase I, Phase II, and Phase III). Only after successfully navigating these phases can a drug enter the market and begin circulating. Even once it’s on the market, it must still pass the rigorous hurdle of post-marketing reevaluation. It’s widely acknowledged that this process is highly resource-intensive, time-consuming, and fraught with numerous obstacles. In fact, every successful new drug development can be likened to Xuanzang’s arduous quest for Buddhist scriptures—requiring years of perseverance, enduring countless trials and tribulations, and ultimately achieving success only through unwavering determination and resilience. According to statistics, developing a single new drug internationally usually takes around 10 years on average and costs approximately $1 billion. And that’s not even counting the many promising candidates that tragically fail along the way. After all, if any stage in the drug development pipeline—whether it’s early-stage research, clinical testing, or post-market evaluation—produces unfavorable results, the entire drug development effort could come to an abrupt halt.
As a type of cutting-edge innovative medicine, stem cells face even greater challenges. Currently, stem cell therapy for ischemic stroke is still only in the clinical trial phase, meaning it’s still some time away from being available on the market. Exactly how long that will take? Well, it’s really hard to say!
Don’t worry—while there aren’t yet any stem-cell therapies available on the market for treating ischemic stroke, patients already experiencing severe long-term complications still have the option to volunteer for clinical trials and get an early chance to try out this cutting-edge technology.
For detailed methods, see: Quick tip: How can patients enroll in stem cell clinical trials and gain access to free treatment opportunities?
Because stem cell sources are complex, and each institution employs different preparation processes and quality-control standards, this can significantly impact treatment outcomes. Therefore, when selecting clinical trials to join, participants should pay close attention to the source and manufacturing process of the cells used in the trial.
Currently, several globally renowned companies are developing stem-cell therapies for ischemic stroke, including Athersys in the U.S., Stemedica in the U.S., and ReNeuron in the UK.
Among them, Stemedica of the United States is a company specializing in stem cell research, boasting 14 years of experience in stem cell production and preparation. The company’s low-oxygen-tolerant bone marrow mesenchymal stem cells are cultivated entirely under hypoxic conditions, making them particularly "resilient" in the ischemic environment of the human body.
Among them, Stemedica's clinical trial of bone marrow mesenchymal stem cells for treating ischemic stroke in the U.S. is one of the 17 U.S.-based clinical trials mentioned earlier on stem cell therapies for ischemic stroke.
Jiuzhi堂 is optimistic about the promising future of combining regenerative medicine with traditional Chinese herbal medicine. To capitalize on this opportunity, Jiuzhi堂 initiated the Zhuhai Hengqin Jiuzhi堂 Yonghe Qihang Fund, which has invested in the U.S.-based company Stemedica. At the same time, Jiuzhi堂 established Jiuzhitang Maker—the sole Chinese partner entrusted with handling Stemedica’s cutting-edge stem-cell technology in China.
Jiuzhitang Maker has signed a "Joint Construction Agreement for a Stem Cell Clinical Research Base" with Beijing Tiantan Hospital, Affiliated to Capital Medical University. The base has now been completed, and the two parties will jointly conduct clinical trials targeting conditions of mutual interest, such as sequelae from stroke.
Once the trial begins enrolling participants, it could be a great option for patients who meet the eligibility criteria.
Related News