Quickly save this! How can patients enroll in stem cell clinical trials and get the chance for free treatment?
来源:Jiuzhitang Maker Stem Cells
2019-07-29
For patients battling serious illnesses where conventional treatments have reached their limits, enrolling in clinical trials and trying out cutting-edge technologies or innovative new drugs may offer a much-needed lifeline. However, some of these groundbreaking therapies haven’t yet been approved or made available in China, making it incredibly challenging for patients to find relevant trial information. Take stem-cell therapies, for instance—though no such products are currently on the market in China, numerous compelling clinical cases have already emerged in the media, showcasing remarkable therapeutic outcomes for patients who had exhausted all other treatment options. To help those considering participation in stem-cell clinical trials, Xiaomei has gathered comprehensive information, compiling a list of hospitals conducting stem-cell research as well as companies actively developing new stem-cell-based medications.
In 2015, the former National Health and Family Planning Commission and the former State Food and Drug Administration jointly issued a document clarifying the registration system for clinical research involving stem cells. As a result, China’s stem cell field began moving toward a "dual-track" approach—stem cell clinical research governed primarily as a technological innovation, while stem cell clinical trials are regulated more closely as pharmaceutical products.
To find opportunities for free enrollment in treatment programs, you’ll first need to understand the "dual-track system" governing stem cell management.
I. Clinical Research on Stem Cells
Which hospitals are authorized to conduct clinical stem cell research?
In July 2015, the former National Health and Family Planning Commission and the State Food and Drug Administration jointly issued the "Administrative Measures for Clinical Research on Stem Cells (Trial)," which stipulated that Grade-III hospitals must complete both "Registration of Clinical Research Institutions for Stem Cells" and "Registration of Clinical Research Projects for Stem Cells" before they can commence clinical studies—referred to hereafter as the "Dual Registration." In other words, only hospitals that have successfully completed institutional registration are eligible to conduct stem cell clinical research. Furthermore, hospitals can officially start their research only after finishing the project registration in addition to the institutional registration. This measure reflects the nation's enhanced efforts to strengthen regulatory oversight of stem cell clinical research.
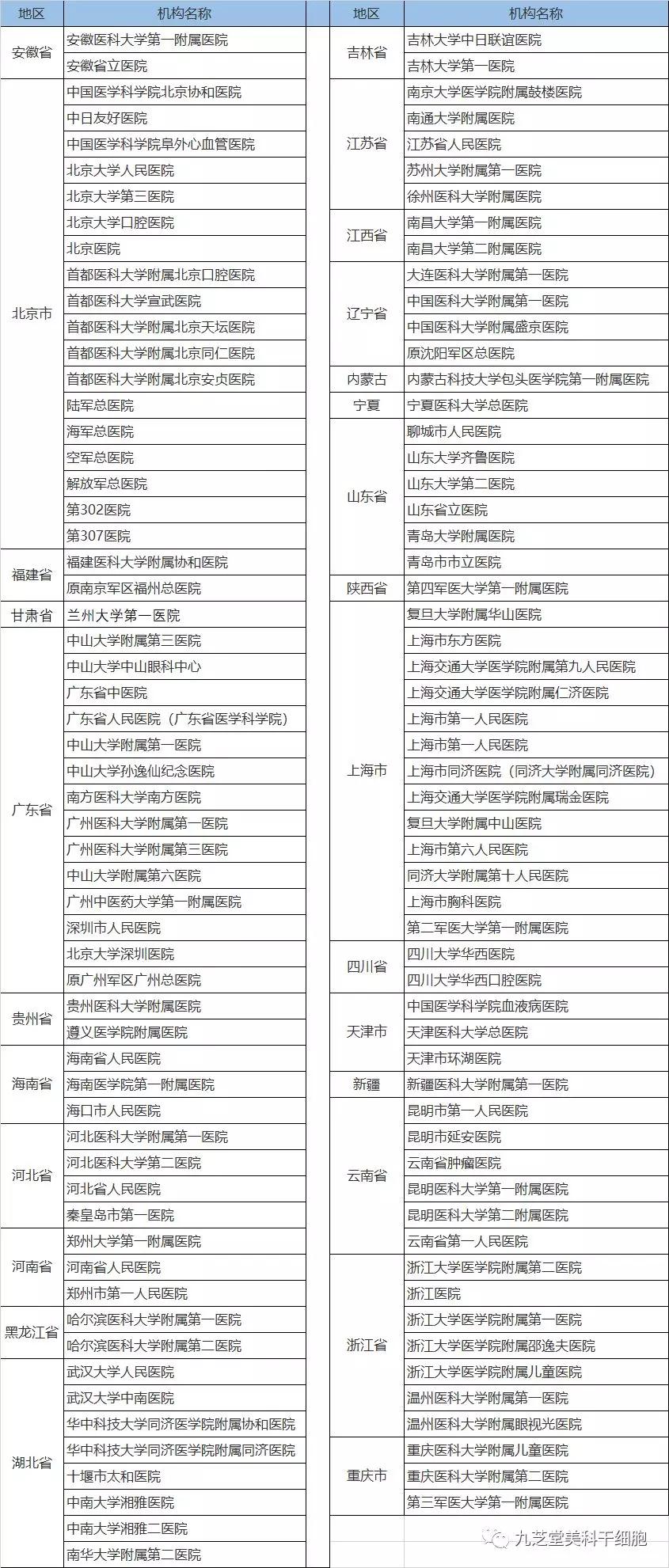
According to the Southern Daily, as of June 2019, the number of local hospitals that have completed registration as clinical research institutions for stem cells increased to 104. Adding the 12 military hospitals already on the list brings the total number of registered facilities to 116. The national government implements dynamic management of these registered institutions, meaning the number may grow or shrink depending on future circumstances. Therefore, if you’re considering joining a clinical study, be sure to choose a hospital that has successfully completed the registration process.
Hospitals That Have Completed Registration as Clinical Research Institutions for Stem Cells
Which diseases are currently being studied in clinical trials?
As mentioned earlier, simply completing institutional registration does not allow stem cell clinical research to begin—furthermore, the specific research project being studied must also be registered.
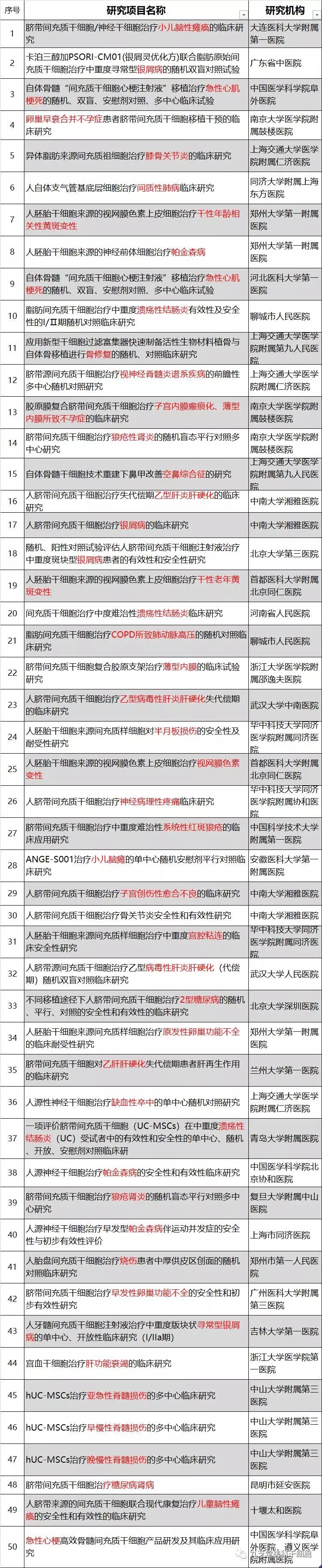
Currently, 51 clinical stem cell studies covering 31 different indications have been officially registered in China, addressing conditions such as gynecological disorders, eye diseases, digestive system issues, orthopedic conditions, neurological disorders, cardiovascular and cerebrovascular diseases, endocrine system disorders, and autoimmune diseases.
Stem Cell Clinical Research Project
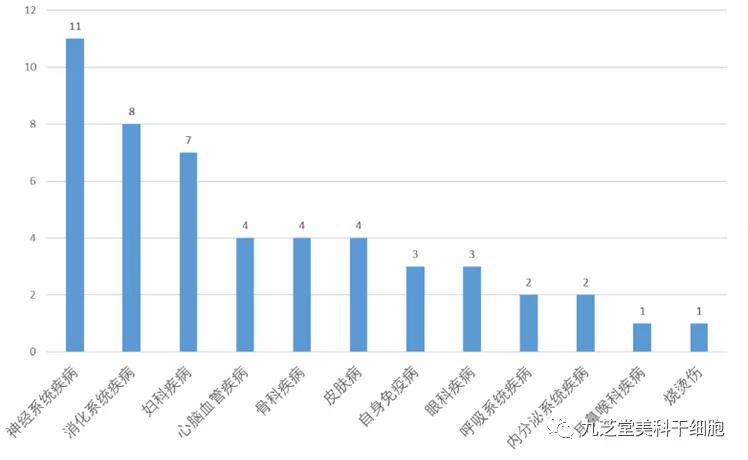
The stem cell clinical research project involves disease classification.
Currently, only 51 out of 116 research institutions have completed the required registration for their studies. Institutions that have already registered stem cell clinical research projects but have not submitted any new project filings by the end of 2020 must resubmit their institutional registration materials. Failure to do so in a timely manner will be considered an automatic waiver of their existing registration. As a result, it is expected that more clinical research projects will continue to be registered in the future.
What cells are used in the registered clinical research project?
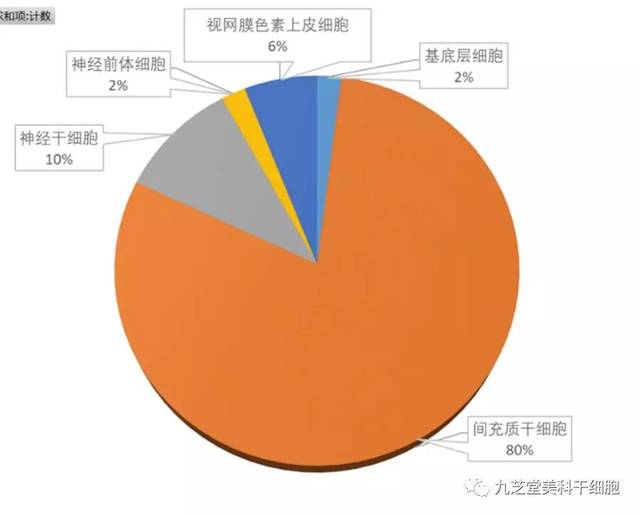
The registered clinical research projects involving stem cells cover a variety of cell types, including mesenchymal stem cells, neural stem cells, neural precursor cells, retinal pigment epithelial cells, and more. Among these, mesenchymal stem cells are the most commonly used, with 40 research projects specifically focused on them—accounting for as much as 80% of all stem cell-related studies.
Statistical Overview of Cell Types in Stem Cell Clinical Research Registration Projects
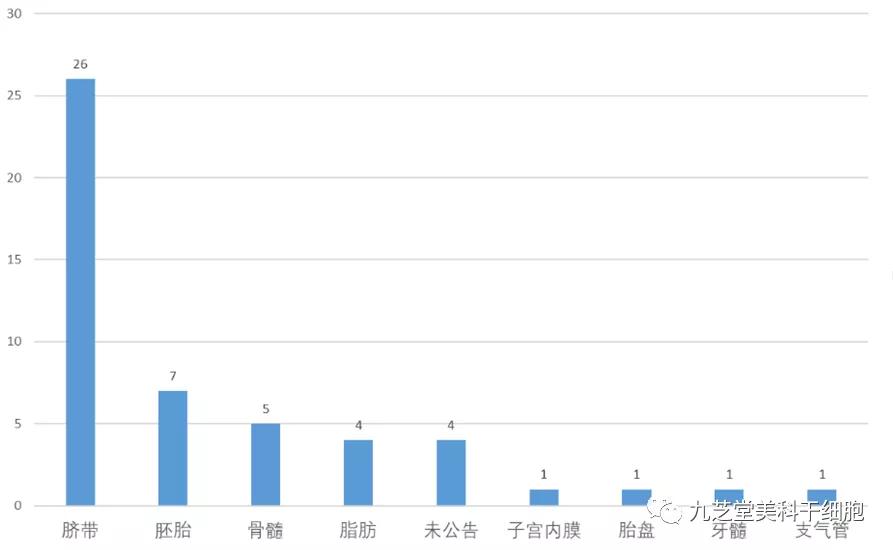
From an organizational perspective, umbilical cord is the primary tissue source, accounting for 26 research projects—more than 50% of the total. Embryos, bone marrow, and adipose tissue are also common sources, contributing a combined 16 projects.
II. Clinical Trials with Stem Cells
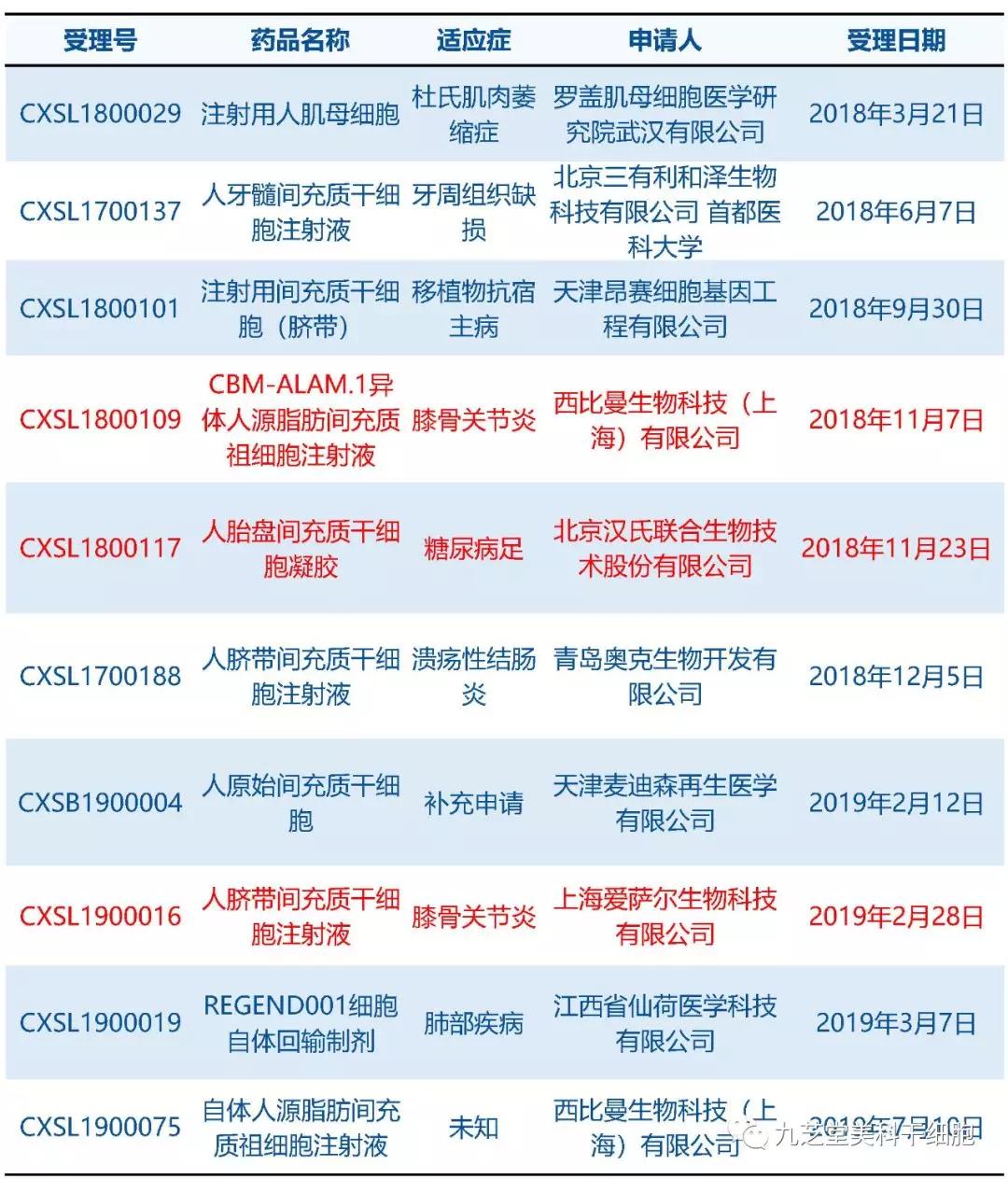
As of July 18, 2019, the National Center for Drug Evaluation has received 10 clinical trial applications for stem cell-based new drugs, three of which have already been approved to proceed with clinical trials. As shown in the table below, projects marked in red are those that have received approval (excluding clinical trials that expired prior to 2018). These studies were initiated by pharmaceutical companies; if you're interested in participating in any of these clinical trials, please contact the companies directly for more information.
The National Medical Products Administration's Center for Drug Evaluation has already accepted clinical trial applications for new stem-cell drugs.
What qualifications are required to join a clinical trial?
Clinical trials typically set inclusion and exclusion criteria. For example, participants may be limited based on the type of disease they have or the stage of their condition, and requirements might also apply to factors like gender, symptoms, and medical history—only those who meet these criteria are eligible to join the trial.
Second, you should also determine whether the trial aims to cure the disease, slow its progression, or simply reduce side effects. Additionally, consider what phase the trial is in—and whether it aligns with your specific needs. For instance, Phase 1 trials focus on establishing a safe dosage, while Phase 3 trials are designed to evaluate whether the new treatment is more effective than existing ones. Clearly identify what you’re looking for, and then check if it matches the trial’s intended purpose.
The inclusion criteria for each clinical trial vary, so it’s important to contact the researchers or check the clinical study registry website for details. Compare your own situation against these criteria to see if you qualify—and when in doubt, consult your doctor for clarification.
Exclusion criteria typically set for clinical studies involving stem-cell therapies usually include the following (these are just examples):
1. Severe allergic constitution;
2. Positive screening results for HBV, HCV, HIV, syphilis, and other infections;
3. Combined malignant tumors and hematologic disorders;
4. Pregnant and breastfeeding women;
5. Patients with severe impairment of liver and kidney function;
6. History of mental disorders, substance/alcohol abuse within the past 5 years; or a history of psychosis that remains uncontrolled and exhibits unstable response to medication.
Where can I find enrollment information, and how do I get in touch?
You can find information on registered research projects—such as inclusion criteria, exclusion criteria, and contact details of relevant medical institutions—by visiting the Chinese Clinical Trial Registry website (www.chictr.org.cn) or the Drug Clinical Trial Registration and Information Disclosure Platform (www.chinadrugtrials.org.cn). For projects that are not yet registered, you can directly reach out to the medical institution or pharmaceutical company conducting the trial for more details.
Related News