Cell therapy may restore normal development in fetuses with spina bifida—three babies born after receiving prenatal treatment
2022-10-11
Three infants born with spina bifida have received cell therapy at the UC Davis Health Center in California. Researchers describe this as a groundbreaking clinical trial—the first-ever globally unique approach combining surgery with stem cells, performed while the fetus is still developing in the mother’s womb—potentially improving outcomes for children born with this congenital condition.

The clinical trial launched in spring 2021 and is officially named the "CuRe Trial: Cell Therapy for In-Utero Repair of Myelomeningocele." A total of 35 patients will participate in the study. The first clinical participant is named Emily, whose daughter Robbie was diagnosed with spina bifida while still in her mother's womb. This birth defect can lead to a range of lifelong complications affecting cognition, mobility, as well as urinary and bowel functions—and it’s typically detected through ultrasound screening.

Preliminary work has shown that combining prenatal surgery with human placenta-derived mesenchymal stromal cells, secured onto a biocompatible scaffold to form a "patch," can help lambs born with spina bifida walk again. When the sheep offspring treated with stem cells were born, they were able to stand up and even run around almost normally. Later, a pair of English Bulldogs became the world’s first dogs successfully treated using this innovative surgical and stem-cell therapy combination.
This time, the research team created a stem cell patch for Emily’s fetus. During the procedure, they gently "floated" the fetus into position at the incision site, carefully placed the stem cell patch directly onto the exposed spinal cord, and then closed the incision to promote tissue regeneration. After the baby was born, doctors were thrilled to see Robbie already kicking—despite the fact that she was originally expected to be born with leg paralysis.
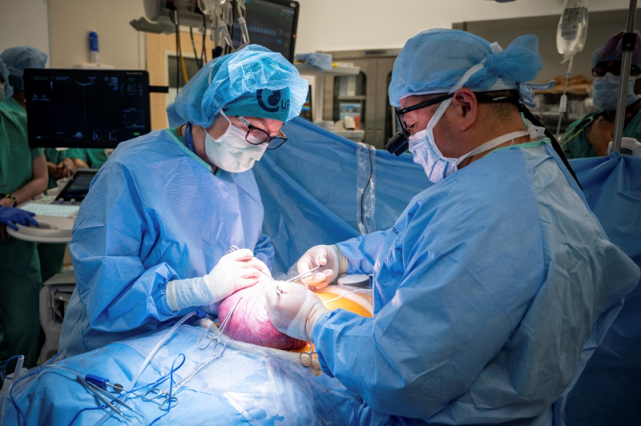
"This experience has exceeded everyone’s expectations. I hope this trial will improve the quality of life for many more patients in the future," Emily said. "We’re honored to be part of history."
"The lead researcher of the study, Diana Farmer, a professor and chief of surgery at the UC Davis Health Center, said, 'I’ve been working toward this day for 25 years.'"
The team remains cautious about the final conclusions and notes that there is still much to study at this stage of the trial. They will closely monitor the three infants who have already been born as part of the clinical trial, tracking their development until they reach 30 months old, in order to fully assess the safety and effectiveness of the treatment.
Reprint Notice: This article is reprinted from Science and Technology Daily. If there are any issues, please contact us for removal.
Related News




