The "Ethical Assessment Guidelines for Stem Cell Sources," co-drafted by Jiuzhitang Maker, has been officially released.
2021-11-26
On November 25, 2021, the group standard "Ethical Assessment Guidelines for Stem Cell Sources" was officially released! Jiuzhitang Maker was invited to serve as one of the drafting units, with Chairman Zhang Quancheng and General Manager Gao Yansong participating in the drafting process.
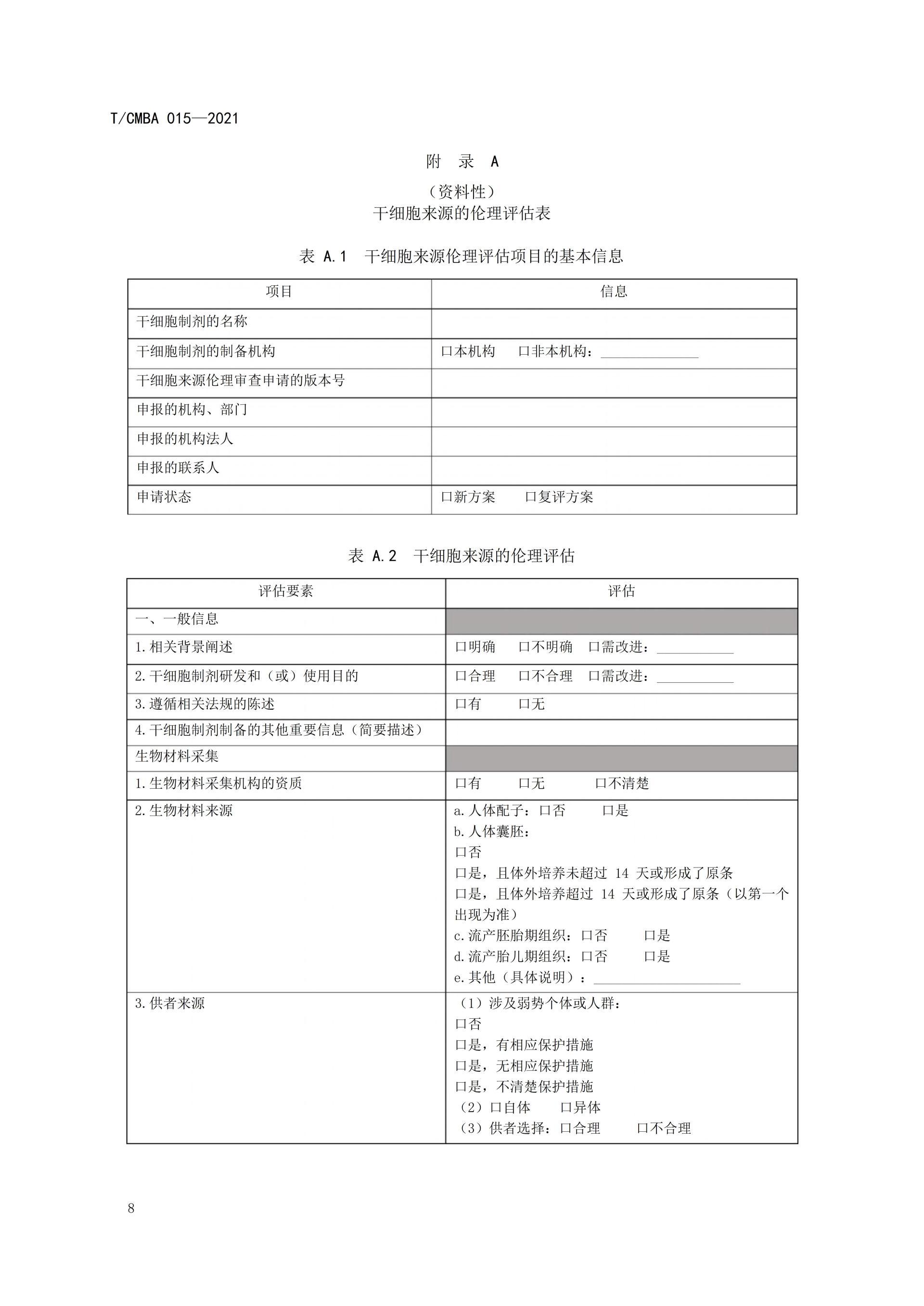
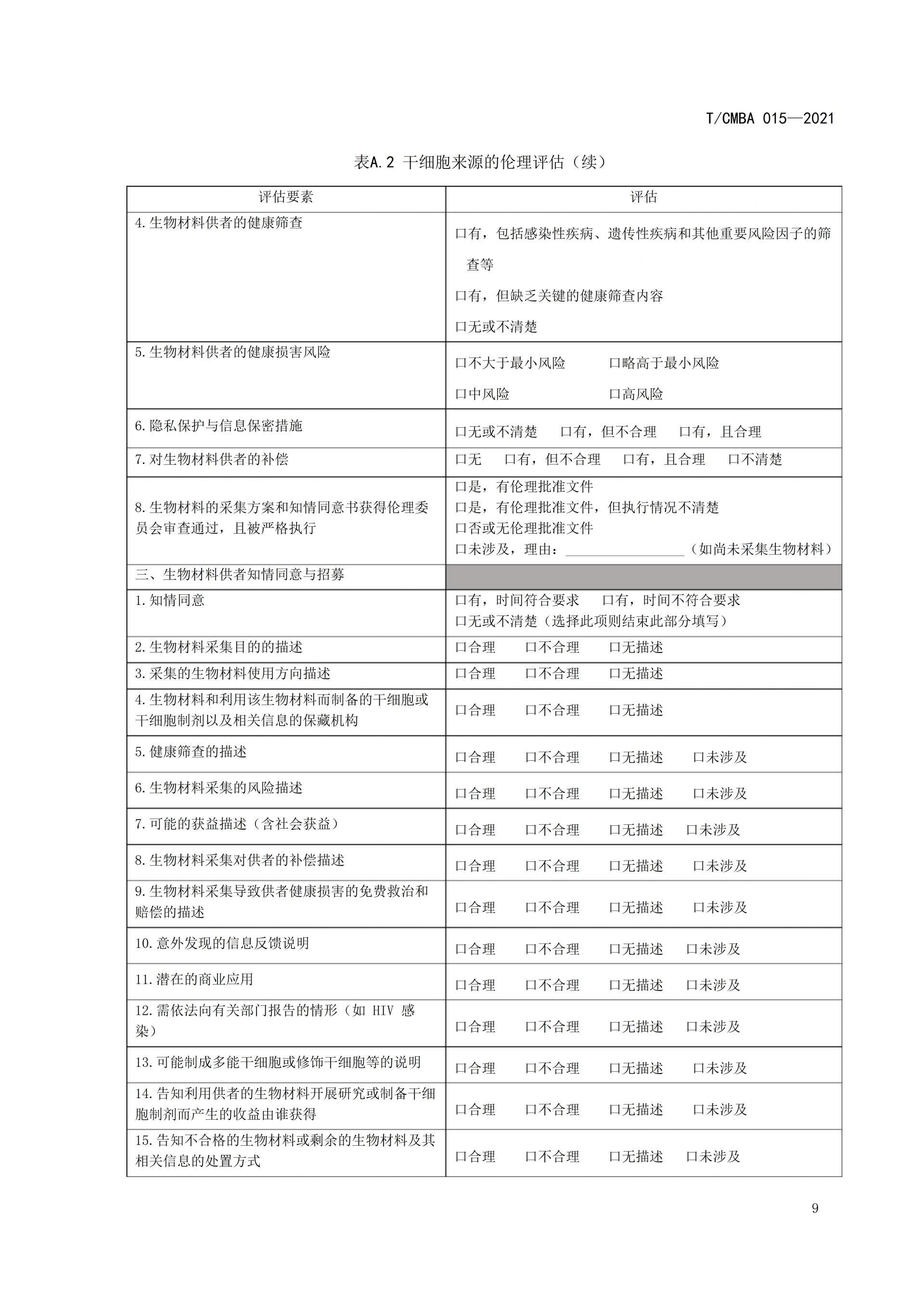
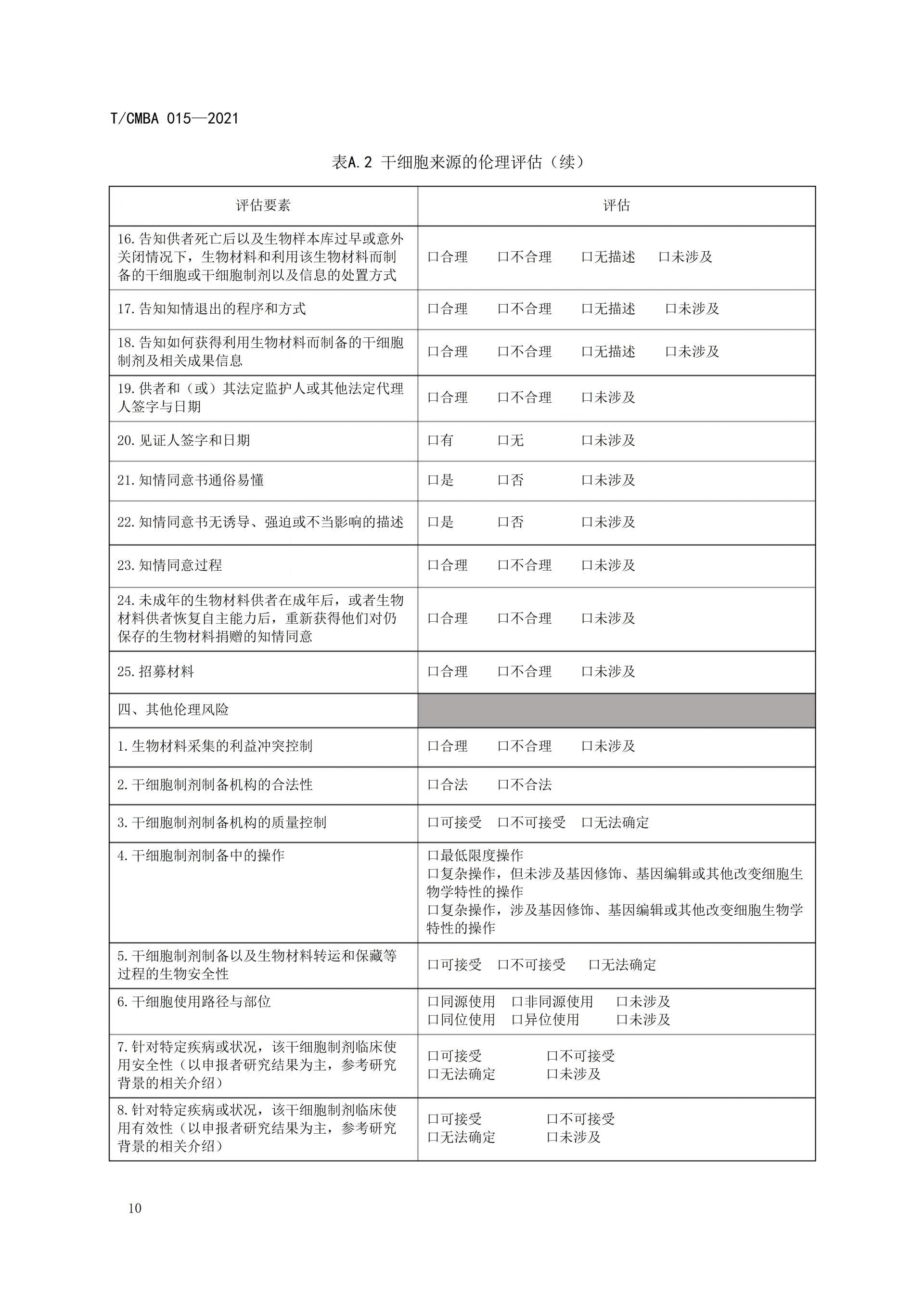
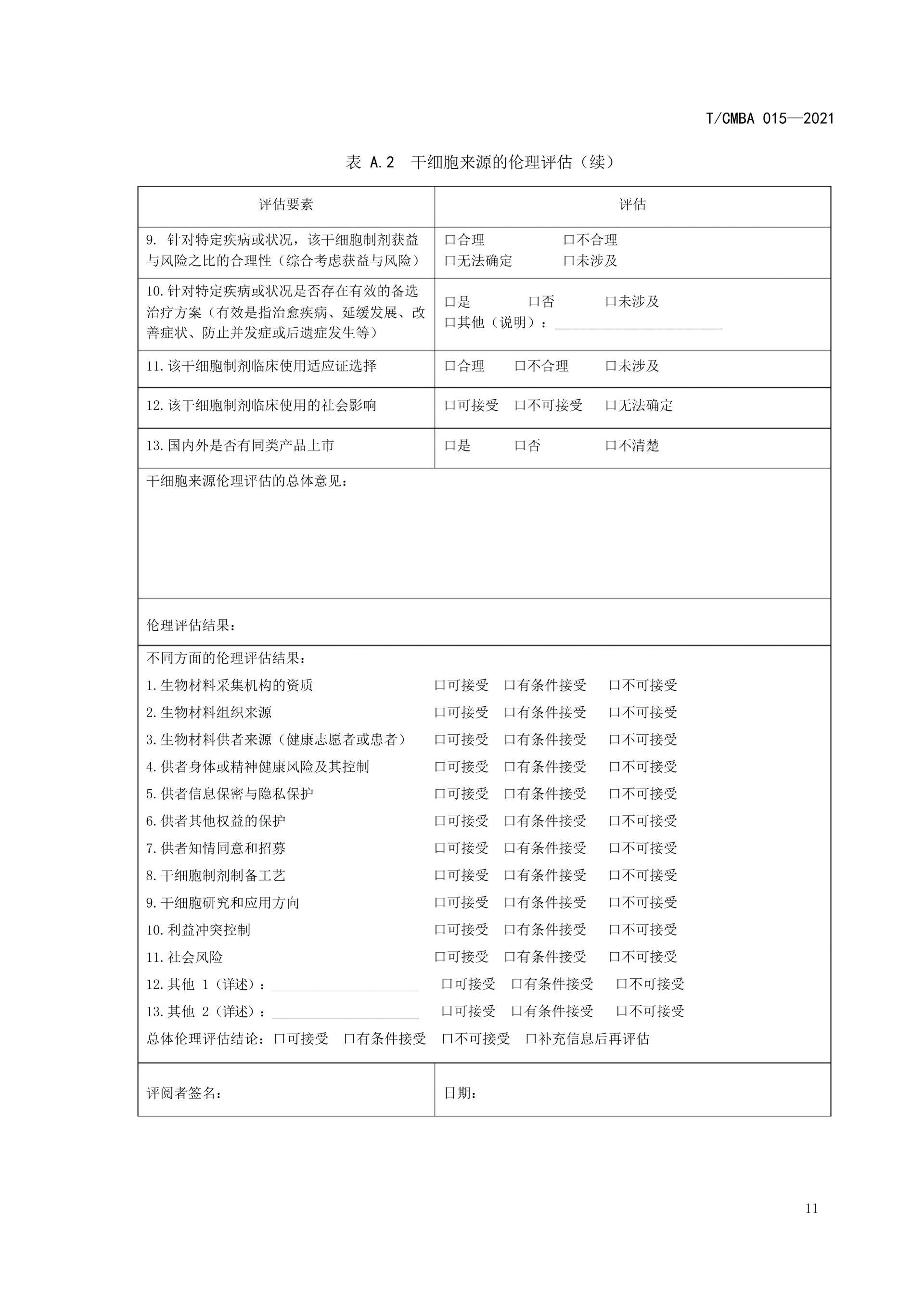
This guideline was spearheaded by the China Association of Medical Biotechnology, bringing together leading institutions and experts in the field. Drawing on relevant guidelines from other countries as well as China's existing regulations and standards, it was jointly developed after multiple rounds of thorough discussions. The aim is to provide guidance for organizations involved in conducting ethical assessments of stem cell sources, ensuring that the collection of bio-materials derived from stem cells and the subsequent preparation of stem cells adhere to ethical principles and regulatory requirements. This effort seeks to prevent unethical stem cell technologies and their clinical applications from causing adverse impacts or long-term harm to both humans and society, ultimately fostering self-regulation within the industry.
Only two companies were invited as drafting units this time: Fosun Pharma and Jiuzhitang Maker. As a leading player in the stem cell industry, Jiuzhitang Maker is actively participating in the development of the group standard, further highlighting its technological strength and influence in the stem cell field. Moving forward, Jiuzhitang Maker will continue to lead industry advancements, playing its part in fostering the sector's sustained and healthy growth!












Previous:
Related News




