Stem Cells: A New Tool to Conquer Aplastic Anemia
2020-06-15
Did you know? Every year, June 14 is celebrated as World Blood Donor Day. The date was chosen because it marks the birthday of Karl Landsteiner, the Nobel Prize-winning scientist who discovered the ABO blood group system. At the heart of World Blood Donor Day is recognition of the life-saving gift donated by voluntary, unpaid blood donors. In 2020, the campaign’s theme was “Safe Blood Saves Lives.”

Blood and blood product transfusions save millions of lives each year—whether it’s women experiencing life-threatening hemorrhages during pregnancy and childbirth, children suffering from severe anemia due to malaria and malnutrition, patients with blood and bone marrow disorders, aplastic anemia, hereditary hemoglobin disorders, or immune deficiency diseases, or victims of trauma, emergencies, disasters, accidents, and individuals requiring surgical procedures.
Although the demand for blood and blood products is widespread, securing safe blood and blood derivatives remains a significant challenge, with safety often difficult to guarantee and inventory levels critically low. Today, countries around the world are urgently seeking innovative solutions to address this critical imbalance between supply and demand.
Today, let’s take aplastic anemia as an example to explore new directions in treating blood disorders.
What is aplastic anemia?
Aplastic anemia (AA) is a condition characterized by the failure of hematopoiesis, leading to a reduction in all types of blood cells. The underlying causes of aplastic anemia may include a deficiency of hematopoietic stem cells, abnormalities in the bone marrow microenvironment, and immune system dysfunction—and crucially, the abnormalities affecting progenitor cells, stromal cells, and the immune system in the same patient do not occur independently of one another. Of the [3] 。 The inherent heterogeneity of aplastic anemia patients determines the complexity of their underlying disease mechanisms, which is why single-drug therapies for AA often yield limited clinical efficacy.
Currently, treatments for AA include both supportive therapy and targeted therapy. Supportive therapy primarily involves anti-infection treatments, sterile environment protection, component blood transfusions, and the use of hematopoietic growth factors such as GM-CSF and G-CSF—tools aimed at preventing and managing complications associated with low blood cell counts. For severe aplastic anemia, intensified immunosuppressive therapy is the treatment of choice. On the other hand, targeted therapy focuses on replenishing or replacing severely depleted or damaged stem cells, such as through allogeneic hematopoietic stem cell transplantation or allogeneic bone marrow mesenchymal stem cell therapy, with the ultimate goal of restoring independent hematopoietic function.
What are the differences between hematopoietic stem cells and bone marrow mesenchymal stem cells?
To put it vividly, if we think of hematopoietic stem cells/bone marrow mesenchymal cells as a towering tree, then bone marrow mesenchymal stem cells would be the tree’s roots. From a differentiation perspective, hematopoietic stem cells are the adult stem cells of the blood system, capable of giving rise to various blood cell types—such as red blood cells, white blood cells, and platelets. In contrast, bone marrow mesenchymal stem cells, originating from the mesoderm, are also adult stem cells with an even greater capacity for self-renewal and multi-directional differentiation. They can transform into a wide array of tissue cells, including vascular, osteogenic, chondrogenic, adipogenic, neural, and muscular cells. Beyond providing mechanical support to hematopoietic stem cells (HSCs) in the bone marrow, these mesenchymal stem cells also secrete multiple growth factors—such as IL-6, IL-11, LIF, M-CSF, and SCF—to actively promote and sustain hematopoiesis. [1] 。
Yue Han and colleagues from the Institute of Hematology, Chinese Academy of Medical Sciences, the Institute of Hematology & Blood Diseases Hospital, and the State Key Laboratory of Experimental Hematology have conducted a preliminary study on the biological characteristics of bone marrow mesenchymal stem cells in patients with aplastic anemia. [1] It was found that AA MSCs compared to the normal control group.
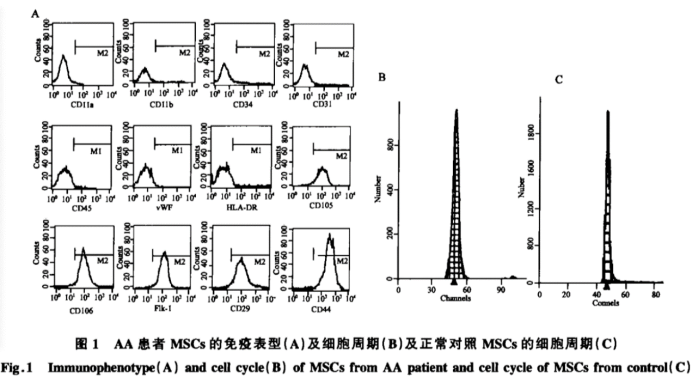
In AA patients, MSCs tend to differentiate more readily into adipocytes, leading to a reduction in their ability to further differentiate into osteoblasts. This shift also results in a diminished HSC niche, potentially disrupting the hematopoietic microenvironment characteristic of AA patients. Not only does this fatty transformation reduce the number of HSC niches in the bone marrow stroma, but studies also show that the surface adhesion molecules on these cells undergo significant changes—collagen expression decreases, while hyaluronic acid expression increases. Such alterations may directly impair the interactions between stromal cells, thereby affecting the proliferation and differentiation of hematopoietic stem and progenitor cells—and ultimately contributing to the pathogenesis of AA.
1. Bone marrow fatty infiltration is the most prominent pathological feature of AA bone marrow.
2. AA is pancytopenia caused by hematopoietic failure. Its pathogenesis is complex, with abnormalities in the bone marrow's hematopoietic microenvironment impairing blood cell production even in the early stages.
Bone marrow mesenchymal stem cells offer new hope for patients with aplastic anemia.
In recent years, with the deepening of stem cell research and the growing number of cases benefiting from bone marrow mesenchymal stem cell transplantation, treatment options for aplastic anemia have shown promising new possibilities.
Sun Limin and colleagues published "Clinical Observation on the Impact of Bone Marrow Mesenchymal Stem Cell Therapy on Bone Marrow Hematopoietic Function." [2] The article states that 12 patients with severe primary aplastic anemia were clinically observed and treated with intravenous infusion of bone marrow mesenchymal stem cells, yielding the following results:
1. After about 3 months of treatment, the interval between blood transfusions significantly increased in 12 patients compared to before treatment.
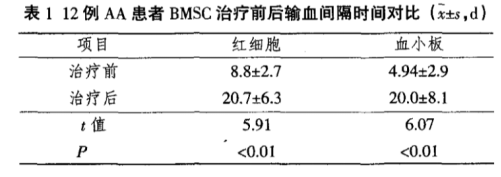
2. Peripheral blood white blood cells, lymphocytes, and platelets showed significant recovery, with differences reaching statistical significance (P < 0.05).
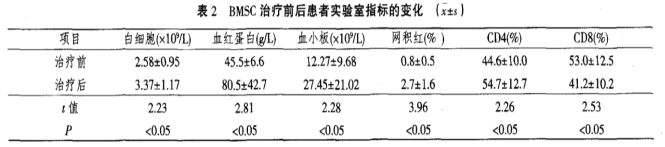
3. There were no significant changes in the patient’s liver function indicators (ALT and total bilirubin) or kidney function indicators (blood urea nitrogen and creatinine) before and after treatment, with differences not reaching statistical significance (P > 0.05).
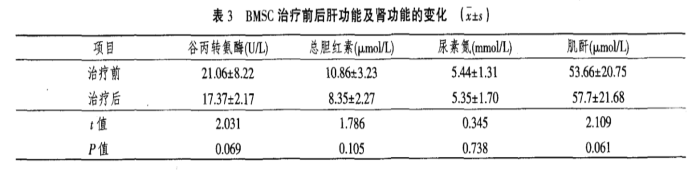
4. The CD4/CD8 ratio in patients improved significantly before and after treatment, with a statistically significant difference (P < 0.05).

Bone marrow mesenchymal stem cells are a type of non-hematopoietic stem cell found in the bone marrow, characterized by their potent immunomodulatory effects. They can effectively regulate hematopoiesis, preventing fat cells from replacing hematopoietic cells in the bone marrow of AA patients. By improving the hematopoietic microenvironment in AA patients, these cells ultimately promote bone marrow-mediated blood cell production.
Ischemia-Tolerant Human Bone Marrow Mesenchymal Stem Cells Is The rising star among bone marrow mesenchymal stem cells
Research shows that ischemia-tolerant human bone marrow mesenchymal stem cells (it-hMSCs), produced under fully hypoxic conditions, exhibit significantly enhanced proliferation, homing, tissue-repair, and anti-inflammatory capabilities compared to stem cells cultured in normoxic environments. As illustrated in the figure below, it-hMSCs demonstrate greater sensitivity to key cytokines involved in wound healing—such as EGF, bFGF, VEGF-121, IL-1β, IL-6, and TNF-α—resulting in improved homing ability.
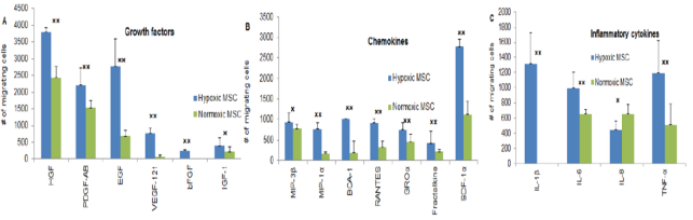
MSCs cultured under normal oxygen conditions also exhibit some anti-inflammatory regulatory capacity, while it-hMSCs derived from hypoxic culture demonstrate even stronger anti-inflammatory abilities. As a result, these hypoxia-preconditioned MSCs are better equipped to play a pivotal role in the treatment of aplastic anemia, offering new hope for patients suffering from this challenging condition.
Jiuzhitang Maker has acquired the core technology for scalable, standardized, and traceable stem cell production by introducing Stemedica Corporation of the U.S.'s globally leading, end-to-end hypoxic clinical-grade stem cell preparation platform.
Jiuzhitang Maker has established Beijing’s first large-scale allogeneic stem cell R&D and production facility in the China Pharmaceutical Valley—Daxing Biomedical Base of Zhongguancun Science Park in Beijing, adhering to cGMP standards compliant with China, the U.S., and the EU. Currently, Meike has completed the technology transfer, enabling mass production of it-hMSCs. Jiuzhitang Maker will now provide highly active it-hMSCs to support the treatment of aplastic anemia.
References:
1. Yue Han, Chen Lei, Li Jing, and Zhao Chunhua, "Preliminary Study on the Biological Characteristics of Bone Marrow Mesenchymal Stem Cells in Patients with Aplastic Anemia" (State Key Laboratory of Experimental Hematology, Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences), China Biotechnology Journal, 2005, 25(6): 20–24
2. Sun Limin, Chen Lingzhen, and Wang Cuilin, "Clinical Observation on the Impact of Bone Marrow Mesenchymal Stem Cell Therapy on Bone Marrow Hematopoietic Function," Contemporary Nurses, 1006–6411 (2012) 09–0001–02
3. Xiong Tao, Liang Yan, Liu Min, Yu Zhuojun, Zhang Liming, and Huang Zhiping from the Department of Hematology at Jingzhou Central Hospital—Clinical Study on Bone Marrow Mesenchymal Stem Cell Therapy for Chronic Aplastic Anemia. International Medical & Health Guide News, Vol. 24, No. 14, July 2018
Related News




