Does reintroducing allogeneic stem cells introduce someone else's genes into the body? What are the potential risks?
2020-02-26
What is a gene? Simply put, in biology, a gene is a unit of genetic information—specifically, a DNA or RNA sequence that carries hereditary instructions. During the life cycle of a cell, this genetic information stored in the DNA sequence is transcribed and translated into biologically active protein molecules. (As we mentioned in a previous article, if you think of a cell as a manufacturing plant, then genes are essentially the production blueprints.)
Further reading: "Aging Is a Cellular Decision"
Genes aren’t formed in a day or two, nor do they simply appear out of thin air—they’re the result of plants and animals’ ancestors steadily accumulating traits over generations as they adapted and evolved.
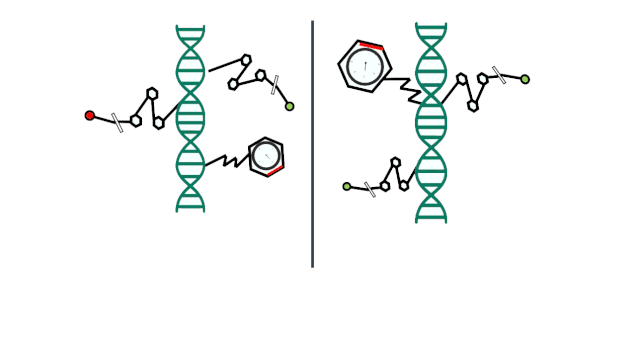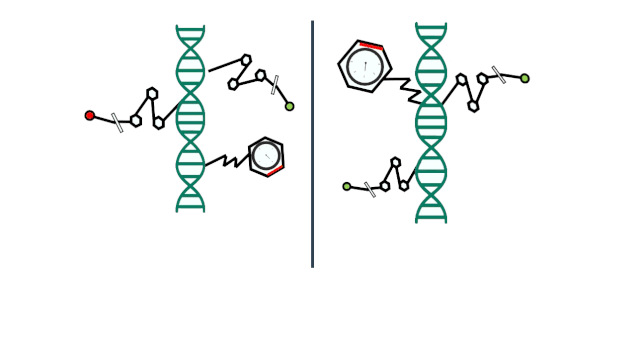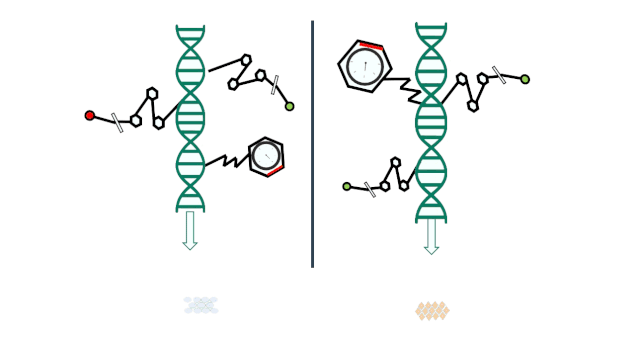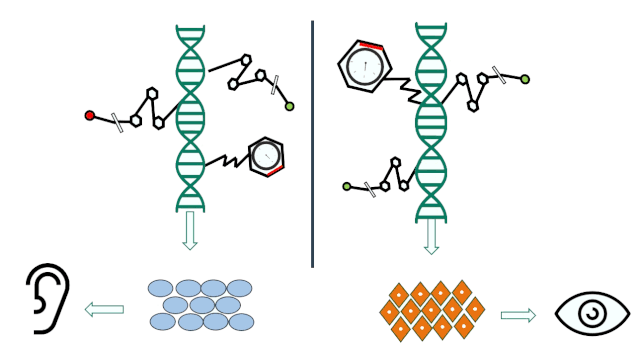
In other words, you’ve inherited some genes from your ancestors, but over the course of your life, your lifestyle, environmental factors, and other experiences have also led to subtle changes in certain genes. While these changes may not be dramatic, they’re certainly not negligible. And because these altered genes are passed on to the next generation, that generation will, in turn, experience their own set of modifications—and the cycle continues like this, generation after generation.
It can be said that all life phenomena—such as birth, growth, decline, illness, aging, and death—are closely linked to genes.
In this day and age, anyone can spit a tube of saliva to reveal their genetic "past lives," decoding the "factory settings" inherited from their ancestors and parents—this refers to genetic testing, which we won’t delve into further in this article.
What are we concerned about when introducing someone else's genes into our bodies?
Actually, this question is asked very broadly—here are three points the editor has summarized:
✎ Genetic information has been tampered with (with another person's genes present in your own body or that of your descendants)
✎ Disease-causing genes pose health risks (cancer and other hard-to-treat genetic disorders)
✎ Could two different DNA molecules within the same organism lead to conflicts?
First, let’s address the first point. To begin with, we need to confirm that what’s being reintroduced are living, viable cells—cells whose genes reside inside the cell itself. In our own bodies, most of our genetic material is housed in the cell nucleus. When foreign DNA enters a cell, it first makes its way into the cytoplasm, where the substances capable of dissolving DNA are also located. Given how intricate and tightly regulated this biological process is, the likelihood of our own genome being inadvertently altered by an external cell is extremely low.

Of course, nothing in life is absolute—viruses are a prime example of this. They’re incredibly cunning, able to evade the immune system’s surveillance by cleverly integrating their own genetic information into host cells, thereby ensuring their survival and reproduction. That’s why stem cell therapies must undergo rigorous quality control checks to confirm they’re free from viral or bacterial contamination—and, crucially, from any risk of cancerous transformation—before they can safely be used in clinical settings.
As for concerns about whether disease-causing genes could be introduced into the body via cell transplantation, potentially posing certain risks, we touched on this issue in a previous article. Even normal cells must first clear the immune system's scrutiny—so how much more challenging would it be for cancer cells carrying faulty genes?
Further reading: "Can Cancer Be Contagious? These Cases Might Shake Your Beliefs."
In the rare cases where cancer develops as a result of organ transplantation, a key reason is that transplant recipients must take immunosuppressive drugs to suppress their own immune systems from rejecting the donor organ—allowing cancer cells to thrive unchecked.
The logic behind stem cell transplantation and organ transplantation is actually quite similar. Take bone marrow (hematopoietic stem cell) transplantation as an example: because there’s concern about immune rejection—known as "graft-versus-host disease"—after the transplant, matching is essential before proceeding with the procedure.
Just like with organ transplantation, the risks associated with stem cell transplants aren’t due to the presence of someone else’s genes in your body—but rather stem from a molecular structure within the cells called MHC (major histocompatibility complex).
This is a self-checking ability that human cells have evolved—where the results of the check are displayed on the cell surface via the molecular structure of MHC. Immune cells possess the remarkable capacity to distinguish between "self" and "non-self," relying on MHC molecules on the cell surface to carry out their daily surveillance tasks. If an abnormal MHC signal appears on the cell surface, immune cells swiftly respond by eliminating the rogue cell.
The third question: DNA isn’t the direct cause of conflict. As explained earlier, as long as it can perform its normal functions, organ transplantation becomes possible. The real key lies in whether the immune system will accept this "visitor from beyond"—and every year, thousands of people worldwide are already waiting for bone marrow (hematopoietic stem cell) transplants. Clearly, compared to concerns about foreign genes, their top priority is simply staying alive.
Additionally, currently, mesenchymal stem cells are the most widely studied type in clinical stem cell research. As previously mentioned in an earlier article by Cell Kingdom, these cells are rarely "rejected" by recipient cells, a characteristic medically known as low immunogenicity. This makes mesenchymal stem cells an ideal choice for transplantation therapies. Moreover, after fulfilling their therapeutic role, they are gradually replaced by newly formed, native tissue cells—meaning they don’t linger in the body long-term.
Further reading: "Why Umbilical Cord and Placenta Mesenchymal Stem Cells Don’t Require Matching" "Understanding Mesenchymal Stem Cells in One Article"
With advances in life sciences, many patients with terminal illnesses have seen their lives extended thanks to stem cell transplants. Moreover, the positive effects of stem cells are increasingly being confirmed in research aimed at treating challenging diseases such as diabetes, Parkinson's, and ALS.
According to data from the "2019-2030 Stem Cell Therapy Contract Manufacturing Market Report" released by Research and Markets, the world's largest market research firm, 16 stem cell drugs are already available on the market globally, while nearly 200 candidate stem cell therapies are currently in development—including allogeneic stem cell treatments.
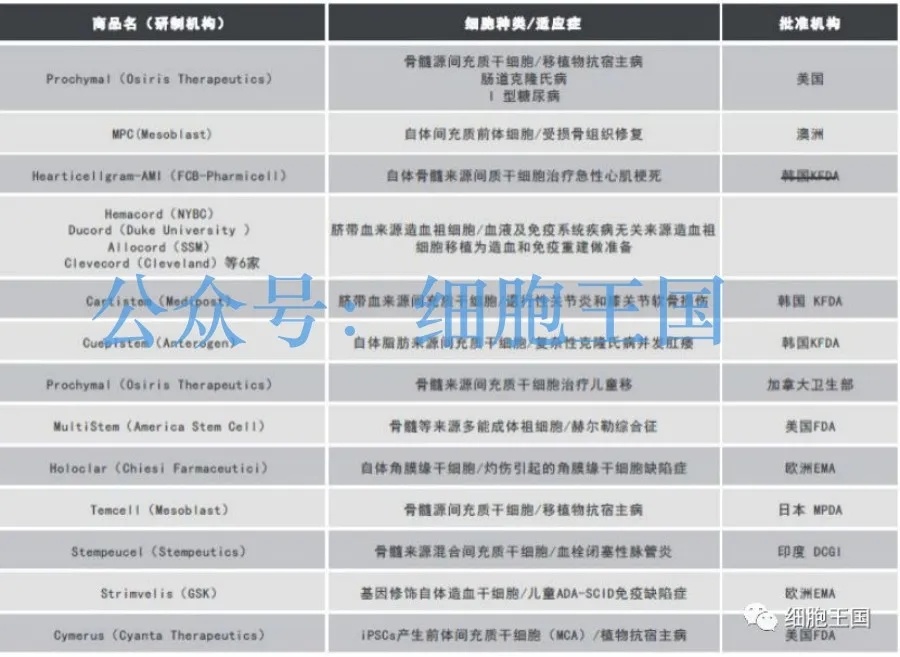
Therefore, rather than worrying about whether reintroducing allogeneic stem cells might introduce foreign genes into the body, we should instead focus more on quality control during cell production, clinical progress stages, and ethical considerations.
Further reading: "Must-Read Before Stem Cell Therapy: Safety Analysis and Quality Control Guidelines for Mesenchymal Stem Cells"
Thanks to advancements in life sciences, humanity now has a deeper understanding of aging and disease—and when it comes to health, there’s absolutely no need to fear these cells. After all, consider the millions of bacteria and microorganisms that naturally reside on and within us (have you ever tried counting how many foreign genes they carry?); once you realize this, the mystery unravels.
References:
Chimera (genetics). Wikipedia
Heather Murphy: When a DNA Test Claims You’re a Younger Man Living 5,000 Miles Away. The New York Times, December 7, 2019
Heather Murphy. The Case of a Man With Two Sets of DNA Raises More Questions. The New York Times. December 12, 2019
How is the probability of a bone marrow match calculated?
Related News




