Stroke Forum: Who Will Achieve the First Breakthrough in Stem Cell Therapy for Stroke?
2019-08-26
For 20 consecutive years, stroke has been the leading cause of death and disability among Chinese residents. Today, the incidence of stroke continues to rise, with an alarming trend toward younger ages at onset. May the light of regenerative medicine shine brightly on the lives of China's 12.42 million stroke survivors living with long-term disabilities—we’re committed to making this vision a reality!
To further advance basic research in the stroke field domestically and facilitate the translation of foundational discoveries into clinical applications, the "3rd China Stroke Basic and Translational Research Summit Forum," hosted by the Chinese Stroke Association, was held on August 24, 2019, in Mudanjiang. The Chinese Stroke Association is one of the most authoritative organizations in China’s stroke community and wields significant influence within the neurology field. This year’s forum drew participation from neurologists representing dozens of renowned hospitals across the country, as well as researchers from leading scientific institutions and academic institutes.
Professor David Wang, Director of the OSF Stroke Center in Peoria, Illinois, USA, and Professor Zhu Dongya, Dean of the School of Pharmacy at Nanjing Medical University, presided over the opening ceremony. Professor Yang Guoyuan, Vice Dean of the Med-X Research Institute at Shanghai Jiao Tong University, delivered a speech. Dr. Hu Baoyang, Executive Director of the Preparatory Institute for the Innovation Academy of Stem Cell and Regenerative Medicine, Chinese Academy of Sciences, and Executive Deputy Dean of the School of Medicine, University of Chinese Academy of Sciences, as well as Professor Zhu Jianhong from the Department of Neurosurgery at Huashan Hospital affiliated with Fudan University, presented a comprehensive overview of stem cell therapy and an analysis of the current status of clinical research on stem cell treatments for neurological disorders, respectively.
Professor Guoyuan Yang, Vice Dean of the Med-X Research Institute at Shanghai Jiao Tong University, delivers a speech.
Dr. Baoyang Hu, Executive Director of the Preparatory Institute for Stem Cell and Regenerative Medicine Innovation at the Chinese Academy of Sciences, and Executive Vice Dean of the School of Medicine at the University of Chinese Academy of Sciences, delivers a speech.
Professor Jianhong Zhu from the Department of Neurosurgery at Huashan Hospital, Fudan University, delivers a speech.
Ischemia-Tolerant Mesenchymal Stem Cells Become a Highlight of the Forum
Dr. Michael Levy, a professor at the University of California San Diego School of Medicine and Chief of Pediatric Neurosurgery at Rady Children’s Hospital-San Diego, was invited by the Stroke Association Organizing Committee to deliver a keynote presentation titled "The Role of Mesenchymal Stem Cells in the Treatment of Stroke." During his talk, Dr. Levy reviewed the unique biological characteristics of mesenchymal stem cells and further highlighted the distinct features of ischemia-tolerant mesenchymal stem cells cultured under hypoxic conditions—specifically, how these cells exhibit enhanced resilience to oxygen deprivation. This unique trait not only minimizes tissue damage caused by hypoxia but also reduces cell apoptosis while effectively suppressing inflammation. Moreover, these cells demonstrate remarkable tolerance to ischemic environments, promoting the differentiation of neural precursor cells into neurons within the ischemic striatum. By continuously recruiting proliferative cells and fostering neurovascular remodeling, they hold significant promise for the treatment of ischemic stroke.
Professor Michael Levy, Chair of Pediatric Neurosurgery at Rady Children's Hospital in San Diego and Professor of Medicine at the University of California, San Diego.
Professor Michael Levy shared his recently completed Phase I/IIa clinical study as the principal investigator, focusing on the use of mesenchymal stem cells to treat post-stroke sequelae. This study is a clinical trial evaluating allogeneic bone marrow-derived mesenchymal stem cells for the treatment of ischemic stroke-related disabilities, with participants receiving a single intravenous infusion of these allogeneic cells. The research also assesses both the safety of the treatment and its clinical efficacy in improving patients' neurological and motor function impairments.
This study enrolled 36 patients, and continuous multi-parameter assessments conducted after treatment confirmed its safety, with no serious adverse events reported. Six months post-treatment, patients showed significant improvements in several key measures, including the Barthel Index for Activities of Daily Living (BI), the Mini-Mental State Examination (MMSE), the National Institutes of Health Stroke Scale (NIHSS), and the Geriatric Depression Scale (GDS). Notably, among participants whose baseline Barthel scores fell between 95 and 100, the proportion improved dramatically—from 11.4% before treatment to 27.3% at 6 months, and further increasing to 35.5% by 12 months post-treatment. These findings preliminarily demonstrate that intravenous infusion of ischemic-tolerant human bone marrow mesenchymal stem cells (it-hMSC) is a safe and effective approach for treating long-term sequelae of stroke.
The paper related to this research has been accepted by "Stroke," a renowned academic journal in the stroke field, and is expected to be officially published around November.
This clinical trial utilizes it-hMSC, which is manufactured by the U.S.-based company Stemedica under the FDA-approved GMP system. Stemedica has already been authorized in the U.S. to conduct six clinical trials—covering conditions such as stroke—and has initiated 10 additional trials outside the U.S. Furthermore, after successfully completing a Phase III clinical trial in Kazakhstan for the treatment of acute myocardial infarction, it-hMSC has now received regulatory approval for market launch in that country.
Stemedica's Pharmaceutical Manufacturing License
Who will achieve the breakthrough of zero for stroke stem-cell therapies?
Stroke is the leading cause of death and disability among adults in China, characterized by high incidence, high disability rates, high mortality, and a high recurrence rate. Currently, there are over 12 million stroke patients in China, with approximately 2 million new cases reported each year. Notably, 70% to 80% of stroke survivors face long-term disabilities that leave them unable to live independently. Currently, stroke treatment primarily focuses on the acute phase; however, once six months have passed, recovery outcomes tend to decline significantly—or even plateau altogether. By the time patients enter the chronic or post-stroke phase, available therapeutic options are severely limited, offering little more than physical therapy to aid recovery. This leaves many patients enduring lifelong suffering, while placing an immense burden on families and society as a whole.
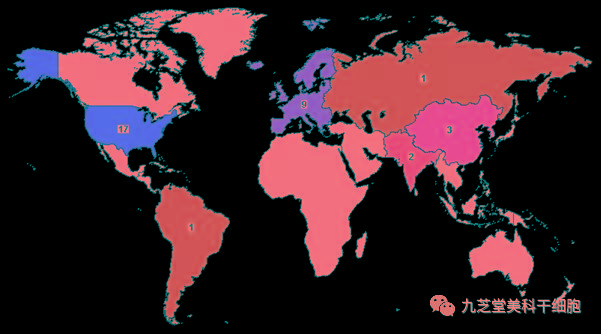
As a specialized treatment for stroke, stem cells have demonstrated promising therapeutic outcomes. According to data from the ClinicalTrials.gov website, as of now, a total of 35 Phase I, II, and III clinical trials focusing on stem cell therapy for ischemic stroke have been conducted worldwide—17 in the United States, 9 in Europe, 3 in China, and 6 in other countries. Notably, Stemedica’s clinical trial is among these ongoing studies.
Geographic Distribution Map of Clinical Trials for Stem Cell Therapy in Ischemic Stroke
Although 14 stem-cell drugs have already been approved for global markets, there are still no stem-cell therapies specifically targeting cardiovascular and cerebrovascular conditions. Globally, most clinical trials exploring stem-cell treatments for stroke remain in Phase I or Phase II. As industry watchers eagerly anticipate which company will break the current zero milestone and become the first to deliver a stem-cell-based treatment for stroke, the race is heating up.
Jiuzhitang Maker (Beijing) Cell Technology Co., Ltd. is transferring the core stem cell preparation technology from U.S.-based Stemedica to China and has signed a "Joint Construction Agreement for a Stem Cell Clinical Research Base" with Beijing Tiantan Hospital. Together, they will jointly conduct clinical trials for indications such as stroke. Looking ahead, Jiuzhitang Maker plans to collaborate with Stemedica in the U.S. on Phase III clinical trials, accelerating the process of bringing stem cell-based drugs to market in China.
Related News