You're invited to the Seminar on Cell-Based Drug Development and Regulatory Submission
2024-03-22
As pharmaceutical supervision With the continuous advancement of institutional reforms in regulatory frameworks, cell therapy products have now clearly been placed on the pathway for pharmaceutical regulation. As of today, five immune cell therapy products have already been approved for market launch in China, while nearly 100 stem-cell-based drug candidates have received implicit approval from regulators and are advancing into clinical trial phases. Meanwhile, numerous companies are actively preparing to submit applications for new cell-based therapies.
The Cell Drug R&D and Regulatory Submission Workshop, hosted by the China Association of Medical Biotechnology and co-organized by Jiuzhitang Maker (Beijing) Cell Technology Co., Ltd., will be officially held in Beijing on April 25–26, 2024. The workshop will feature industry experts who will share insights into the development journey and regulatory pathways for bringing cell therapy products to market—providing invaluable guidance to help more cell therapy companies enhance their drug-approval capabilities and boost success rates, ultimately driving the efficient and standardized growth of the cell therapy industry.
Conference Organizer
China Association of Medical Biotechnology
Co-organizing the conference
Jiuzhitang Maker (Beijing) Cell Technology Co., Ltd.
Meeting time
Check in on April 24, 2024; conference scheduled for April 25–26.
Meeting location
Beijing Guangxi Building (No. 26, Hawei Li, Panjiayuan, Chaoyang District, Beijing)
Meeting Agenda
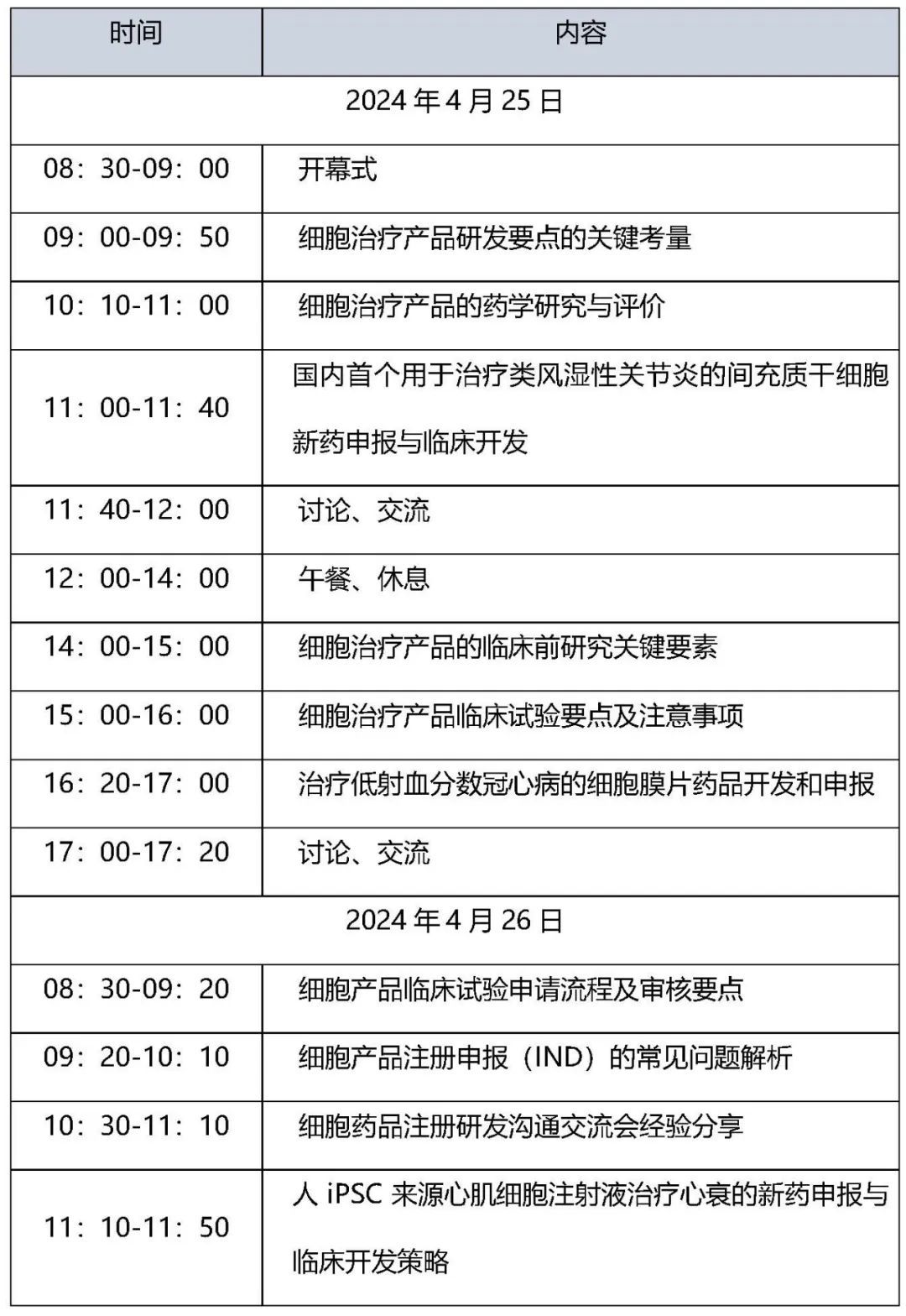
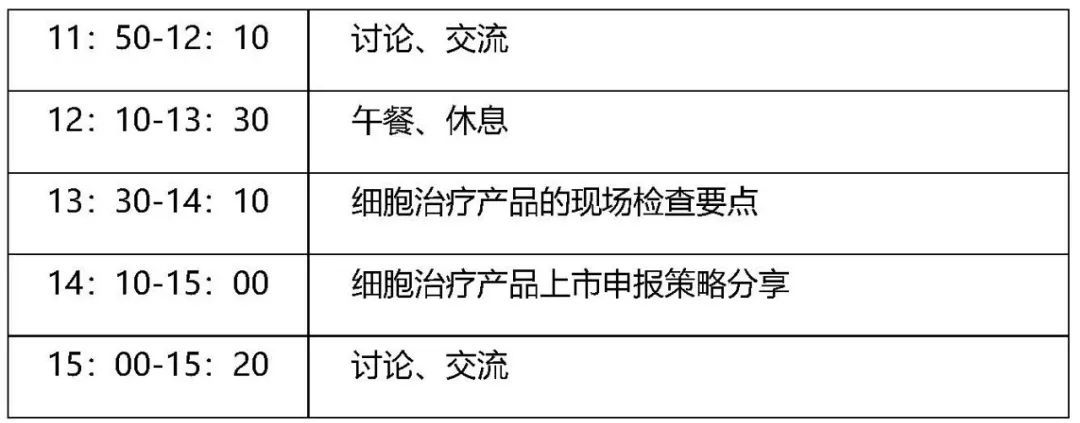
Meeting participants
Quality leaders, production managers, R&D directors, drug registration specialists, and personnel involved in drug product development, manufacturing, quality control, and other activities related to drug submissions.
Meeting expenses
2,980 RMB per person; 2,580 RMB per person for association members. The fee includes instructor fees, venue costs, materials, as well as buffet and tea breaks during the conference.
Remittance account
Bank of Account: Bank of China, Guohua Building Branch, Beijing National Cultural and Financial Cooperation Demonstration Zone
Account Name: China Association of Medical Biotechnology
Account: 324656017253
Please indicate "Cell Therapy Drug R&D and Regulatory Submission Workshop" when making the remittance. Member organizations, please also include your organization's name.
* Bank transfer payment: Deadline is April 23, 2024. Please inform the conference team promptly after transferring the funds so they can confirm receipt.
* Invoices will be issued uniformly upon conference registration.
Conference Registration
Please fill out the registration form and send it to the contact's email address. After making the bank transfer, kindly forward the payment receipt or a screenshot of the bank transfer to the same email address.
Conference accommodation
The conference organizing committee is not responsible for arranging accommodation for participants; please make your own hotel reservations in advance. At the Beijing Guangxi Building, the special rate for this training session is 550 RMB per day per large bed room (including one breakfast), and 600 RMB per day per standard twin room (including two breakfasts). Participants who require accommodation are strongly advised to book their hotels well ahead of time. When making your reservation, be sure to mention "China Association of Medical Biotechnology Conference" to qualify for the agreed-upon rates.
Contact: Zou Yuheng
Contact number: 18500156987
Meeting coordination
Contact: Liang Suli
Contact numbers: 13720054624, 010-62115986-612
Email: liangsl @cmba .org.cn
Related News




