Jiuzhitang Maker Receives Funding Support for the Daxing District Project Promoting the Transfer and Commercialization of Scientific and Technological Achievements
2021-02-26
On February 25, 2021, Jiuzhitang Maker received investment support for its project promoting the transfer and commercialization of scientific and technological achievements, as well as two grants: one for public rental housing rent assistance.
In October 2020, to implement the "Provisional Measures for Promoting the Transfer and Transformation of Scientific and Technological Achievements in Daxing District (Revised in 2020)" and accelerate the conversion of various scientific and technological achievements into real-world productivity within Daxing District, the Daxing District Science and Technology Commission launched a project solicitation initiative aimed at boosting the transfer and transformation of these innovations. The program will prioritize support for technology-based enterprises located in the district, providing them with robust backing as they work to become leaders in science and technology innovation.
Jiuzhitang Maker actively responded to the government's call, mobilizing its research teams to apply for this project—and successfully secured its inclusion. The list of supported projects was publicly announced from November 10 to 16, 2020.
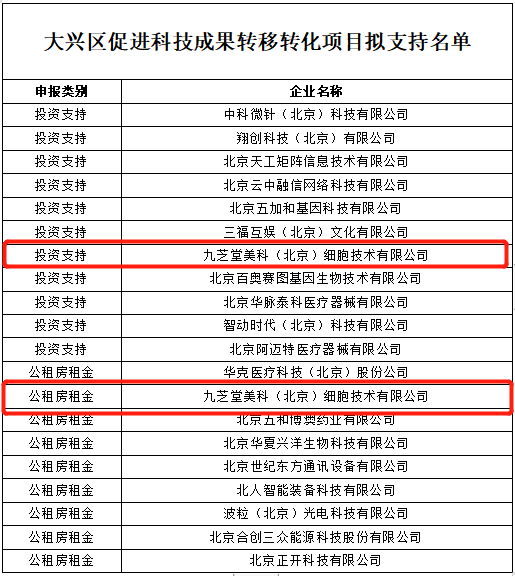
Jiuzhitang Maker boasts a leading production platform and cutting-edge technologies in the stem cell field, dedicated to translating scientific advancements into practical applications. In 2018, as the sole Chinese partner for Stemedica, a global pioneer in stem cell preparation technology based in the U.S., Jiuzhitang Maker successfully introduced Stemedica's world-class, clinical-grade stem cell manufacturing platform. This collaboration enabled the company to master core technologies that ensure scalable, standardized, and traceable stem cell production—breaking through longstanding barriers in the pharmaceutical industry's approach to stem cell manufacturing and filling a critical gap in China's domestic market.
On February 19, 2020, Jiuzhitang Maker and Beijing Tiantan Hospital, Affiliated to Capital Medical University, jointly received approval for their clinical trial application of a new stem-cell-based drug. This milestone achievement marked "three industry firsts" in China's stem-cell pharmaceutical sector: it was the first clinical trial to utilize imported stem cells; the first to target a major neurological indication with stem-cell therapy; and, most notably, the first trial approved by the National Medical Products Administration (NMPA) after it resumed accepting applications for stem-cell clinical trials—specifically, the first to employ bone marrow-derived mesenchymal stem cells. This breakthrough holds landmark significance for the development of China's stem-cell industry.
The clinical trial officially kicked off on January 12, 2021. This is the first stem-cell therapy clinical trial for cerebrovascular diseases conducted in accordance with the new version of the "Good Clinical Practice Guidelines for Drug Clinical Trials," since the country began regulating stem cells as pharmaceuticals. The trial holds significant importance, not only for advancing the clinical application of stem cells in the field of cerebrovascular diseases but also for potentially transforming the entire management system governing stem-cell applications.
Jiuzhitang Maker's recent funding support reflects the Daxing District government's recognition of the company's technological capabilities and its strong track record in research-to-commercialization, highlighting the firm's potential to lead industry advancements in the stem-cell pharmaceutical sector.
Moving forward, Jiuzhitang Maker will intensify its research efforts, accelerate the commercialization of scientific and technological innovations, and drive the development of stem-cell-based pharmaceuticals. This initiative will provide critical technological support in the stem-cell field, helping Daxing build the "China Pharmaceutical Valley" brand and establish a biopharmaceutical hub that is "nationally leading and globally first-class."
Related News




