What do you know about male menopause?
2020-10-29
The 50-year-old uncle, who used to be approachable, calm, and always kind to his family—taking full responsibility for all household chores—has recently started snapping at everyone for no apparent reason, feeling irritable and emotionally unstable. Even when he returns home from work, he seems listless and unmotivated, avoiding his usual chores altogether. On top of that, he’s struggling to sleep soundly at night and has been waking up frequently to use the bathroom.
The hospital diagnosed him with "male menopause syndrome."
Do men experience menopause too? Their families find it unbelievable.
Compared to the intense onset of menopause in women, men’s "andropause" seems far more subtle—and often receives insufficient attention and awareness.

What is male menopause?
Male menopause is the transitional period as men move from middle age into old age. It typically begins around age 40 and becomes more prevalent after 60. During this phase, men may experience varying degrees of sexual dysfunction, psychological challenges, hot flashes, difficulty concentrating, memory lapses, and emotional instability—along with other clinical signs and symptoms. These changes can also impact the function of multiple organ systems, ultimately affecting overall quality of life. [1] 。
Life begins in cells, and aging also begins in cells.
The real beginning of aging isn’t the wrinkles around our eyes or the gray hairs on our heads—it’s the aging of the cells within our bodies. Cells are the fundamental building blocks that define life, aging, illness, and death in humans; after all, the condition of each individual cell collectively mirrors the state of the entire body.
The cell theory tells us that the human body is made up of countless cells, and German scientist Rudolf Virchow noted that all pathological phenomena in the organism are rooted in cellular damage. [2] 。
The article "Effect of aging on behaviour of mesenchymal stem cells," published on June 26, 2019, in World J Stem Cells, clearly elucidates that MSC senescence involves three distinct mechanisms: autophagy, oxidative stress, and the production of extracellular vesicles. [3] 。
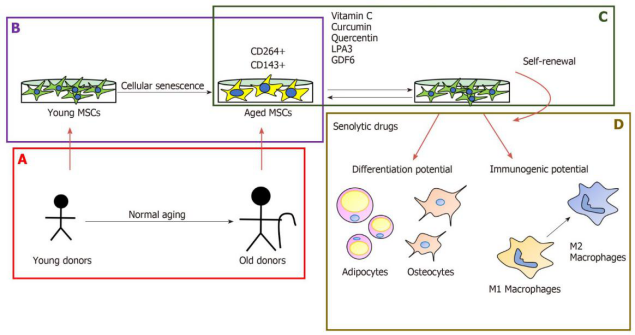
Figure 1: The Effects of Aging on Mesenchymal Stem Cell Self-Renewal, Differentiation, and Immunogenicity.
A, B: Mesenchymal stem cell characteristics: Mesenchymal stem cells (MSCs) are limited by donor age (A) and long-term in vitro culture (B).
C: Some new formulations can mitigate the effects of cellular aging in mice: the therapeutic potential of mesenchymal stem cells;
D: Senolytic treatment affects the behavior of bone marrow mesenchymal stem cells.
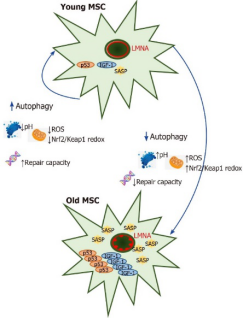
Figure 2 shows how autophagy influences the aging of mesenchymal stem cells.
The self-renewal potential of young mesenchymal stem cells (MSCs) is influenced by their autophytic capacity, which effectively modulates oncogenic factors like p53, as well as the senescence-associated secretory phenotype and inflammatory signaling molecules such as IGF-1. This, in turn, leads to excessive reactive oxygen species production in mitochondria, accumulation of DNA mutations at the genomic level, acidification of the lysosomal apparatus, and increased levels of LMNA in the cell nucleus. When autophagy is downregulated by pathological processes, young bone marrow mesenchymal stem cells rapidly age into senescent bone marrow mesenchymal stem cells, losing their ability to perform autophagy.
Stem cells help men bid farewell to the embarrassment of andropause.
Stem cell applications for intervening in male menopause symptoms primarily manifest in the following four areas:
Stem Cell Therapy for Age-Related Frailty
In October 2017, a Phase II clinical trial published in *The Journals of Gerontology* demonstrated that intravenous infusion of allogeneic mesenchymal stem cells was safe for patients with frailty due to aging. After receiving the stem cell treatment, participants showed significant improvements in key frailty indicators—such as physical performance measures and inflammatory biomarkers. Building on the promising safety and efficacy data from the Phase II trial, the study has now advanced to Phase IIb, with plans to enroll 120 volunteers. [4] 。
Stem cells enhance sexual function
Numerous clinical studies have shown that stem cells are an ideal, health-focused approach to restoring reproductive function, particularly the 2019 study published in *Cell* titled "Evaluation of mesenchymal stem cells in the treatment of infertility in male rats."
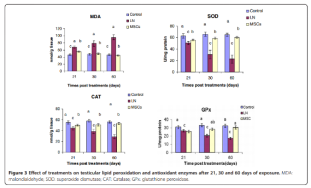
The results showed that testosterone levels and semen quality both improved after treatment with bone marrow mesenchymal stem cells. Additionally, levels of superoxide dismutase, glutathione peroxidase, and catalase were elevated at 21, 30, and 60 days post-MSC treatment. Furthermore, genomic DNA alterations and the percentage of DNA fragmentation were also reduced following MSC therapy. Lead nitrate induced ductal degeneration, necrosis, interstitial edema, and decreased spermatogenic activity; however, these pathological changes in testicular tissue were partially reversed after treatment with bone marrow mesenchymal stem cells. [5] 。
Stem cell therapy for hair loss
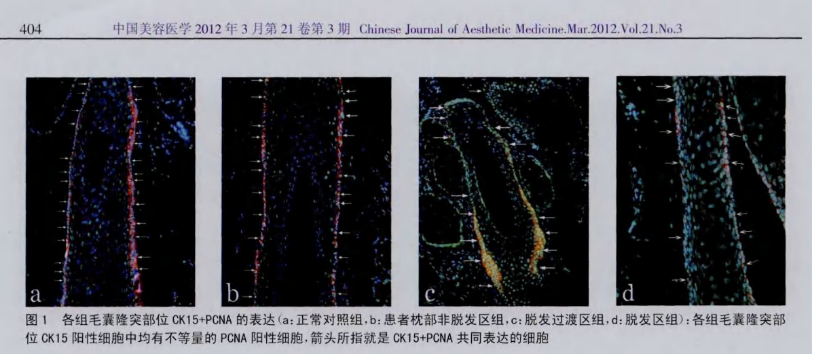
A 2012 study titled "Preliminary Investigation into the Proliferation and Apoptosis of Hair Follicle Stem Cells in Male Androgenetic Alopecia Patients," published in China's Journal of Aesthetic Medicine, revealed the following findings: The number of hair follicle stem cells was identical in both scalp tissues with and without hair loss. However, in scalp tissue from individuals experiencing hair loss, these stem cells failed to generate the progenitor cells necessary for hair growth, indicating that the stem cells themselves had become defective—preventing the scalp from regenerating hair. The study concluded that reduced proliferative activity of hair follicle stem cells likely plays a key role in the progression of male androgenetic alopecia (AGA). Importantly, stem-cell-based therapies now offer a promising new avenue for treating hair loss, providing hope to patients struggling with this condition.
Stem Cell Therapy for Insomnia
The Chinese Journal of Clinical Psychology, Volume 25, Issue 2, 2017, featured the article "Clinical Observation on Intravenous Infusion of Human Umbilical Cord Mesenchymal Stem Cells for the Treatment of Chronic Insomnia." The study aimed to evaluate the efficacy and safety of human umbilical cord mesenchymal stem cell transplantation in treating chronic insomnia. Methods: Thirty-nine patients with chronic insomnia were enrolled and randomly assigned to either a treatment group (n = 19) or a control group (n = 20). Patients in the treatment group received a single session of human umbilical cord mesenchymal stem cell transplantation, while those in the control group were administered oral alprazolam for one month. Both groups underwent clinical assessments using the SF-36 Health Survey Short Form and the Pittsburgh Sleep Quality Index to evaluate treatment outcomes. All participants were followed up for 12 months. Results: Intravenous infusion of human umbilical cord mesenchymal stem cells significantly improved sleep quality and overall quality of life in patients with chronic insomnia. Notably, therapeutic effects were observed as early as one month after transplantation, with benefits lasting up to one year following a single treatment session.
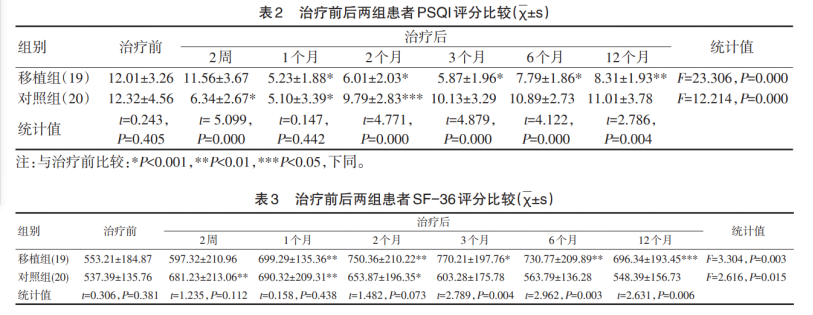
In addition, stem cells have also shown remarkable effectiveness in managing a variety of chronic conditions, including gout, vision and hearing disorders, Alzheimer's disease, knee joint repair, and even depression.
Ischemia-tolerant human bone marrow mesenchymal stem cells deliver significantly better therapeutic outcomes.
Jiuzhitang Maker's ischemia-tolerant human bone marrow mesenchymal stem cells (it-hMSC) are derived from the bone marrow of healthy, young adult donors and are cultured and expanded under fully controlled low-oxygen conditions. Once introduced into the body, these cells demonstrate significantly enhanced therapeutic efficacy. Currently, the company has established a hypoxic stem cell production platform and developed a GMP-compliant stem cell drug system, enabling it to manufacture clinical-grade stem cell products that meet both Chinese and U.S. regulatory requirements for drug submissions.
In February of this year, Jiuzhitang Maker’s clinical trial application to treat ischemic stroke using the product was approved by the Center for Drug Evaluation (CDE) under the National Medical Products Administration, marking a significant milestone for Jiuzhitang in the field of stem-cell-based pharmaceuticals.
itMSCs exhibit superior amplification, homing, tissue-repair, and inflammation-regulation capabilities compared to stem cells cultured under normoxic conditions, as illustrated in the figure below. Specifically, itMSCs demonstrate heightened sensitivity to key cytokines involved in wound healing—such as EGF, bFGF, VEGF-121, IL-1β, IL-6, and TNF-α—resulting in enhanced homing ability.
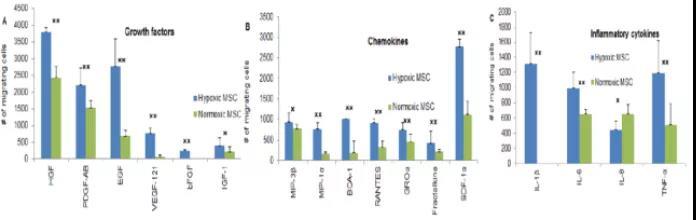
Research shows that MSCs cultured under normoxic conditions also exhibit some anti-inflammatory regulatory capacity, while itMSCs derived from hypoxic culture demonstrate even stronger anti-inflammatory abilities. As a result, these hypoxia-preconditioned cells are better equipped to play a crucial role in combating age-related decline and frailty, making them more likely to safeguard human health effectively.
References:
[1] Guo Yinglu, Li Hongjun. Male Menopause Syndrome[J]. Chinese Journal of Andrology, 2004(08):563-566.
[2] Schultz M. Photo quiz. Emerg Infect Dis [online serial]. Sept 2008
[3] The Effect of Aging on the Behavior of Mesenchymal Stem Cells. World J Stem Cells, June 26, 2019; 11(6): 337–346
[4] Allogeneic Mesenchymal Stem Cells Alleviate Age-Related Frailty: A Phase II Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Gerontol A Biol Sci Med Sci. 2017;72(11):1513-1522.
[5] Evaluation of Mesenchymal Stem Cells in the Treatment of Infertility in Male Rats
Related News




