Details
Alzheimer's disease, commonly known as senile dementia, is a common neurodegenerative disease attacking central nervous system. The pathological features are mainly β-amyloid deposition in the brain, intracellular neurofibrillary tangles and neuron loss. Neuron loss is one of the root causes of functional impairment in Alzheimer's patients. As the disease progresses, patients with severe dementia will become completely dependent on caregivers and experience severe memory loss, with fragments of memory left. Patients will lose the ability of taking care of themselves in daily life and develop gatism, showing silence and limb stiffness. Results of health examination may indicate positive pyramidal tract signs and original reflections such as strong grip, groping and sucking. The patient would slip into coma eventually and usually die of infections and other complications.
With an incidence of 0.71%, there are approximately 10 million Alzheimer's patients in China. Occurrence of Alzheimer’s is closely connected with patient’s age: among population aged 65, about 10% have developed Alzheimer’s, while the percentage among people aged 85 is 50%. As the society ages rapidly, the incidence of Alzheimer's disease will become even higher. It is estimated that by 2050, number of Alzheimer's patients in China will reach 27 million, which will bring a heavy burden to their families and society.
Major pharmaceutical giants have invested billions of dollars into development of drugs for treatment of Alzheimer's disease. However, investigations conducted by consulting agencies show that among 123 drugs co-developed by major pharmaceutical companies between 1998 and 2015, only three drugs and one combination therapy FDA’s marketing approval, and none of these 123 drugs is able to cure Alzheimer's disease, including FDA-approved treatment programs which only work to partially relieve symptoms.
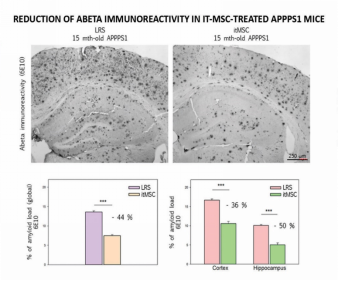
Continuous development of stem cell technologies and advancement of clinical studies on stem cells for treatment of Alzheimer’s have brought hope to people again. As shown by animal studies, after mice were intravenously injected with ischemic-tolerant bone marrow mesenchymal stem cells, the administered cells were detectable in their brains, and β-amyloid deposition in brains was significantly reduced.
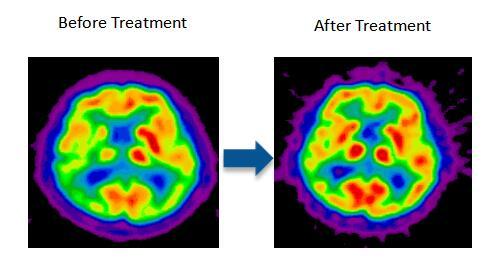
Stemedica's clinical trial on Alzheimer's disease is the first clinical effort using stem cells with US FDA’s approval. The trial was conducted at Emory University and University of California, Irvine. Remarkable efficacy was observed on some patients, who basically returned to normal life after treatment. PETCT scans showed that the cerebral blood perfusion of the patients was significantly improved, as shown in below figure.